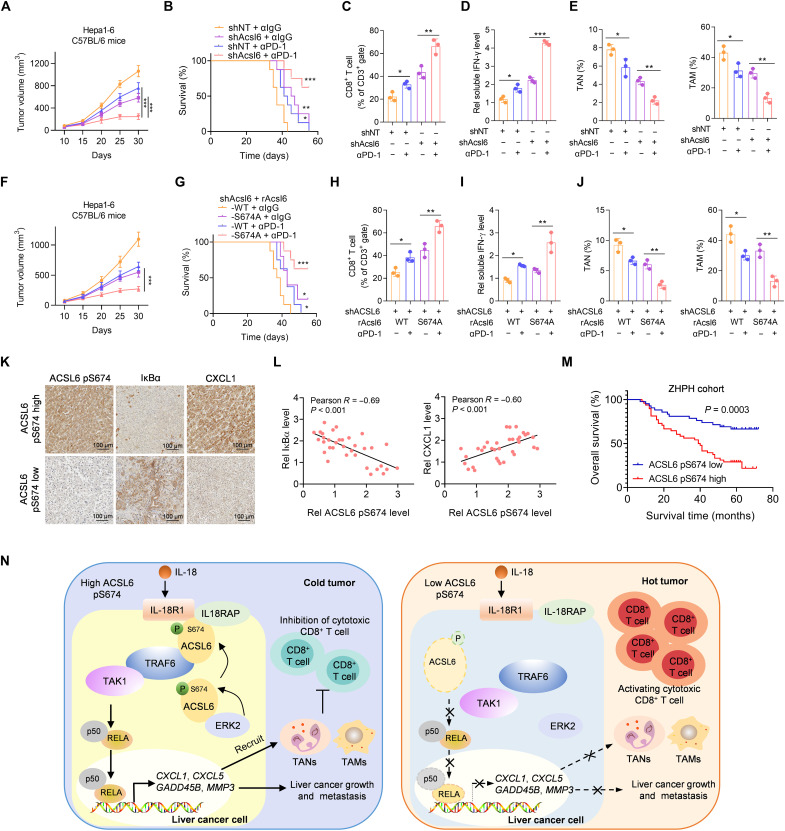
Fig. 8. The level of ACSL6 pS674 negatively correlates with the efficacy of ICIs therapy and the prognosis of patients with liver cancer.
(A to E) Mice with established Acsl6 depletion or control Hepa1-6 tumors were treated with or without anti–PD-1. Tumor volumes (A) and survival rates (B) were recorded. Flow cytometry analyses of tumor-infiltrating CD8+ T cells (C). Measurement of the concentration of IFN-γ (D). Flow cytometry analyses of TANs and TAMs (E). (F to J) Acsl6 WT or S674A was reconstituted into Acsl6-depleted Hepa1-6 cells, which were then subcutaneously injected into C57BL/6 mice, and mice were subsequently treated with anti–PD-1. Tumor volumes (F) and survival rates (G) were recorded. Flow cytometry analyses of tumor-infiltrating CD8+ T cells (H). Measurement of the concentration of IFN-γ (I). Flow cytometry analyses of TANs and TAMs (J). (K and L) IHC staining with anti–ACSL6 pS674, anti-IκBα, and anti-CXCL1 antibodies in tumors from patients with liver cancer from ZHPH (K) and correlation analyses (L). (M) Kaplan-Meier analyses of overall survival according to the ACSL6 pS674 level in patients with liver cancer from ZHPH cohort. (N) Proposed model of the mechanism by which ACSL6 activates IL-18–NF-κB signaling to promote immune evasion and tumor progression in liver cancer. [(A) and (E)] n = 5; [(B) and (G)] n = 10. *P < 0.05, **P < 0.01, and ***P < 0.001. Two-way ANOVA [(A) and (F)], log-rank test [(B), (G), and (M)], and one-way ANOVA [(C) to (E) and (H) to (J)].