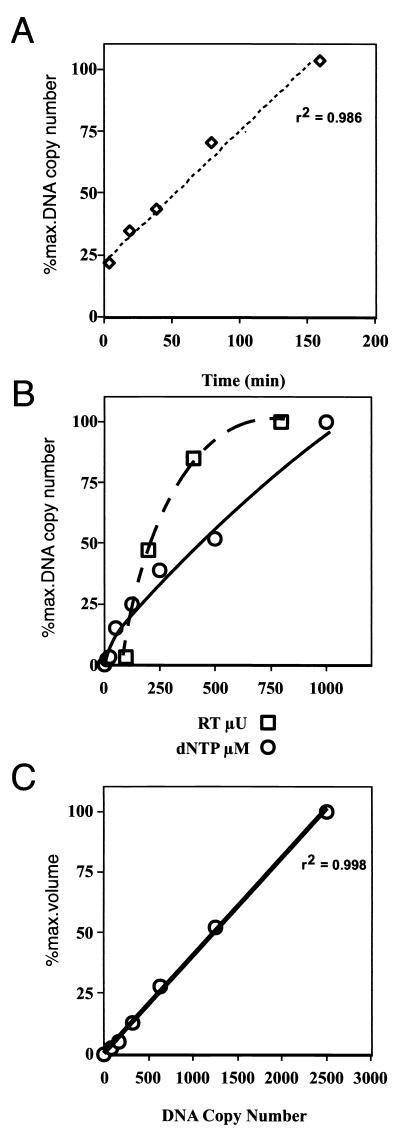
FIG. 2.
Characterization of the NERT-PCR assay. (A) Virus supernatants containing 0.25 mU of total RT activity were incubated with 200 μM total dNTPs at 37°C for the indicated times and assayed for (−)ssDNA by PCR using both proximal (P1 and P2) and distal (D1 and D2 [not shown]) primer pairs (Fig. 1). NERT reaction mixtures were serially diluted, where necessary, to conform to the linear range of the PCR assay. All experiments were performed at least three times with similar results, and results of representative experiments are shown. (B) NERT assays were carried out using 200 μM total dNTPs and increasing quantities of virus supernatant (0.1, 0.2, 0.4, and 0.8 mU of RT activity, measured using a commercial homopolymer template RT assay), or using virus supernatants containing 0.25 mU of RT activity and increasing total concentrations of dNTPs (0, 5, 10, 50, 100, 250, 500, and 1000 μM); these assay mixtures were incubated for 90 min at 37°C, and (−)ssDNA was measured using primers P1 and P2 (Fig. 1). (C) Analysis of control DNAs, used to quantify the PCR results (r2 = 0.998).