Extended Data Fig. 7. Mean (+SD) total REGN5381 concentrations in serum vs time following a single subcutaneous or intravenous injection of REGN5381 in normotensive non-human primates.
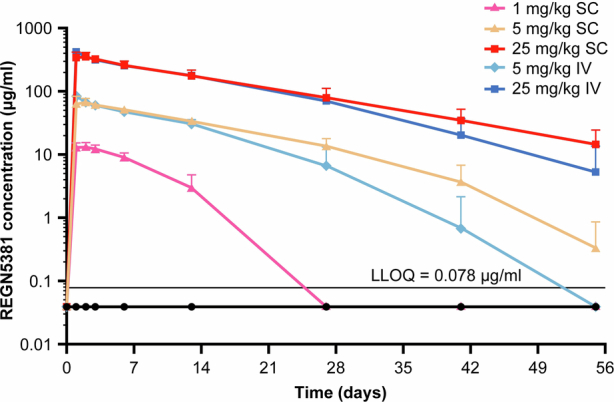
Normotensive cynomolgus monkeys received a single intravenous bolus of saline/vehicle (n = 5; black circles) or REGN5381 subcutaneous (n = 5; 1 mg/kg [pink line]), (n = 5; 5 mg/kg [pale yellow line]), (n = 5; 25 mg/kg [red line]) or intravenous (n = 5; 5 mg/kg [light blue line]) or (n = 5; 25 mg/kg [dark blue line]). Concentrations of total REGN5381 (all drugs; without regard to binding site occupancy) in serum were determined using ELISA. ADA analysis was conducted using a generic immunoassay against anti-human IgG4 antibodies and 32% (8/25) of REGN5381—dosed animals showed a positive response. Nonetheless, all concentration data, regardless of whether potentially impacted by ADA, were included in this plot as the actual exposure would be that used for any correlation with PD effects. ADA, anti-drug antibodies; ELISA, enzyme-linked immunosorbent assay; IgG, immunoglobulin G; IV, intravenous; LLOQ, lower limit of quantification; PD, pharmacodynamics; SC, subcutaneous; SD, standard deviation.
