Abstract
Pulmonary hypertension (PH) is a prevalent complication among patients with chronic kidney disease (CKD). In these patients, pulmonary vasodilators may be useful but are underused. We describe a group of patients with precapillary PH and advanced CKD treated with pulmonary vasodilators. This was a case series of patients with CKD stage 4 and 5 and precapillary PH (isolated or combined) based on right heart catheterization (RHC) treated with pulmonary vasodilators from 2018 to 2023. Of 263 patients with isolated precapillary or combined PH and advanced CKD, only 17 (6%) were treated with pulmonary vasodilators; 53% (n = 9) with precapillary PH and 47% (n = 8) with combined PH. Most patients (94%, n = 16) received phosphodiesterase‐5 antagonists, while 12% (n = 2) received endothelin receptor antagonists. Adverse clinical outcomes were seen in 35% of patients within a year. The use of pulmonary vasodilator did not prevent adverse outcomes in patients with precapillary PH and advanced CKD.
Keywords: chronic kidney disease, precapillary pulmonary hypertension, pulmonary hypertension, pulmonary vasodilator, right heart catheterization
In this case series we describe the clinical characteristics, hemodynamic parameters, and outcomes of patients with CKD stages 4 or 5 with isolated precapillary or combined PH treated with pulmonary vasodilators from a single centre.
INTRODUCTION
Pulmonary hypertension (PH) is a prevalent complication among patients with chronic kidney disease (CKD) that affects approximately 20%–50% of patients. 1 , 2 PH‐CKD is classified as group 5 PH given the several pathophysiologic mechanisms involved, leading to isolated precapillary, postcapillary, and combined PH. 3
CKD can develop as a complication of precapillary PH and RV failure owing to chronic renal venous congestion and neurohormonal dysregulation. 4 Meanwhile, precapillary PH can occur as a consequence of CKD complications as well, including pulmonary artery stiffening (owing to secondary hyperparathyroidism and metastatic calcification), vasoconstriction (owing to an imbalance of vasoactive mediators such as nitric oxide), and vessel remodelling (owing to chronic inflammation and hyperdynamic circulation due anaemia or arteriovenous fistula). 5 , 6 , 7
Although precapillary PH (isolated and combined) has been associated with an increased risk of morbidity and mortality in CKD patients, there is limited evidence supporting the safety and efficacy of pulmonary vasodilators in these patients. 3 , 8 There have been small single‐center studies showing improvement in World Health Organization functional class as well as exercise tolerance. 9 , 10 , 11 Unfortunately, CKD patients have been underrepresented in a variety of cardiovascular clinical trials. 12
We aim to describe the clinical characteristics, hemodynamic parameters, and outcomes of patients with CKD stages 4 or 5 with isolated precapillary or combined PH treated with pulmonary vasodilators.
CASE SERIES
Methods and statistical analysis
This was a single‐center case series of patients older than 18 years with stage 4 or 5 CKD and evidence of isolated precapillary or combined PH confirmed by right heart catheterization (RHC) who were treated with pulmonary vasodilator therapies from 2018 to 2023. Patients with CKD4 had a baseline estimated glomerular filtration rate (eGFR) of 15–29 mL/min, whereas those with CKD5 had an eGFR of <15 mL/min or were on dialysis. 13
We defined pulmonary hypertension (PH) as a median pulmonary artery pressure (mPAP) of >20 mmHg. 14 Isolated precapillary PH was defined as pulmonary vascular resistance (PVR) of >3 wood units and pulmonary arterial wedge pressure (PAWP) of ≤15 mmHg, whereas combined PH was defined as PVR of >3 wood units (or with a transpulmonary gradient (TPG) of >12 mmHg) and PAWP of >15 mmHg. 7 , 15 Severe PH was defined as mPAP of ≥35 mmHg or mPAP of ≥25 mmHg with a cardiac index (CI) of <2 L/min/m. 16
The initiation of pulmonary vasodilator therapy was recorded based on hospital discharge or outpatient follow‐up documentation within a month after the RHC date. Based on chart review, we collected clinical information, CKD characteristics, RHC and transthoracic echocardiography (TTE) data, pulmonary vasodilator therapy features (including medication class, dose, and frequency), and clinical outcomes, including cardiovascular (CV) outcomes or all‐cause mortality within 1 year of follow‐up.
Continuous variables were presented as medians or ranges and interquartile ranges (IQR) when appropriate. Categorical variables were presented in frequencies and percentages or fractions when appropriate. Our study was approved by the Jefferson Institutional Review Board (iRISID‐2023‐2277).
RESULTS
Of 263 patients with advanced CKD precapillary‐PH or combined‐PH confirmed by RHC, only 17 (6%) were treated with pulmonary vasodilators after RHC. Their median age was 60 years (range 39–75), 47% (n = 8) were females, and 88% (n = 15) were African Americans. Most had CKD5 (76%, n = 13), and more than half were on haemodialysis (59%, n = 10). Their baseline comorbidities are presented in Table 1.
TABLE 1.
Clinical data of patients with pre‐capillary or combined PH and advanced kidney disease on pulmonary vasodilator therapies.
| Variables | Total n = 17 |
|---|---|
| Age in years—Median (range) | 60 (39–75) |
| Female sex—n (%) | 8 (47) |
| Race—n (%) | |
| African American | 15 (88) |
| Caucasian | 1 (6) |
| Asian | 1 (6) |
| Body Mass Index—Median (IQR) | 26 (22–32) |
| Kidney disease features—n (%) | |
| CKD stage 4 | 4 (24) |
| CKD stage 5 | 13 (76) |
| On haemodialysis | 10 (59) |
| Median time on dialysis in years (range) | 6 (2–11) |
| Baseline comorbidities—n (%) | |
| Hypertension | 16 (94) |
| Diabetes mellitus | 8 (47) |
| Hyperlipidemia | 9 (53) |
| Heart failure | 10 (59) |
| Chronic lung disease | 6 (35) |
| Obstructive sleep apnea | 2 (12) |
| History of venous thromboembolism | None |
| Connective tissue disease | None |
| History of cancer | 1 (6) |
| Chronic anaemia | 14 (82) |
| PH features—n (%) | |
| Precapillary PH | 9 (53) |
| Combined PH | 8 (47) |
| Severe PH | 14 (82) |
| Non‐severe PH | 3 (18) |
| RHC data—median (IQR) | |
| Mean RA pressure (mmHg) | 16 (9–19) |
| Mean PA pressure (mmHg) | 45 (35–50) |
| PA wedge pressure (mmHg) | 13 (6–24) |
| Transpulmonary gradient (mmHg) | 26 (21–32) |
| Pulmonary vascular resistance (Woods) | 5 (3.3–6.2) |
| Cardiac output (L/min) | 5.1 (4.4–7) |
| Cardiac index (L/min/m2) | 3 (2.4–3.9) |
| Echocardiography data—n (%) | |
| LVEF in %—median (IQR) | 55 (40–60) |
| LV diastolic dysfunction (n = 13) | 10 (77) |
| PASP in mmHg—median (IQR) | 61 (45–73) |
| TRV in m/sec—median (IQR) | 3.3 (3.1–3.7) |
| RV strain | 11 (65) |
| RV dilation | 14 (82) |
| RA dilation | 16 (94) |
| IVC diameter of >2.1 cm | 12 (71) |
| IVC inspiratory collapse of <50% | 11 (65) |
| Pulmonary vasodilator therapies | |
| PDE5 antagonists | 16 (94) |
| Endothelin receptor antagonists | 2 (12) |
| Calcium channel blockers | 8 (47) |
| Single therapy | 9 (53) |
| Combination therapy | 8 (47) |
| Outcomes (1 year follow‐up) | |
| Mortality | 3 (18) |
| Cardiovascular outcomes | 4 (24) |
Abbreviations: CKD, chronic kidney disease; IVC, inferior vena cava; LVEF, left ventricular ejection fraction; PA, pulmonary artery; PASP, pulmonary artery systemic pressure; PDE5, phosphodiesterase 5; PH, pulmonary hypertension; RA, right atrium; RV, right ventricle; TRV, tricuspid regurgitation velocity.
Based on RHC evaluation, 53% (n = 9) had isolated precapillary‐PH, and 47% (n = 8) had combined‐PH. Most patients were classified as having severe PH (82%, n = 14). Regarding echocardiography findings, the median left ventricular ejection fraction was normal (55%, IQR 40–60), whereas all patients had right‐sided abnormalities suggestive of PH. Among patients with isolated precapillary‐PH, 8/9 had diastolic dysfunction on TTE. Complete RHC and TTE data are presented in Tables 1 and 2.
TABLE 2.
Individualized clinical data of patients with pre‐capillary or combined PH and advanced kidney disease on pulmonary vasodilator therapies.
| Demographics | Baseline comorbidities | Kidney disease features | Vasodilator used and dosage | PH type and severity | Baseline RHC data | Follow‐up RHC data | Time between RHC | Echocardiographic data | Clinical outcomes at 1 year follow‐up | |
|---|---|---|---|---|---|---|---|---|---|---|
| Subject 1 | 39 y.o. male, African American | Hypertension | CKD5 on dialysis for 8 years via AVF | Sildenafil 20 mg every 8 h, ambrisentan 5 mg daily |
Precapillary PH Severe PH |
mPAP 39, PCWP 9, PVR 3, CO 10.1, CI 4.9 | mPAP 44, PCWP 1, PVR 7, CO 6.1, CI 2.9 | 3 months | LVEF 55%. DD present. ePASP ~75 mmHg. TRV 3.71. RV strain and dilation present | Stroke. Died 222 days after RHC date |
| Subject 2 | 60 y.o. female, African American | Hypertension, diabetes mellitus | CKD5 on dialysis for 6 years via AVF | Sildenafil 10 mg every 8 h, nifedipine 60 mg daily | Combined PH. Severe PH | mPAP 67, PCWP 16, PVR 10.4, CO 4.9, CI 3.1 | None | ‐ | LVEF 20%. DD present. ePASP and TRV unavailable. RV strain and dilation present | Received kidney transplant during RHC admission. No adverse CV outcomes |
| Subject 3 | 58 y.o. male, African American | Hypertension, heart failure, and ILD | CKD4 not on dialysis. Baseline Cr 4 and eGFR 15 | Sildenafil 20 mg every 8 h |
Precapillary PH Severe PH |
mPAP 45, PCWP 9, PVR 8.4, CO 4.3, CI 2.4 | None | ‐ | LVEF 65%. DD present. ePASP ~100 mmHg. TRV 4.45. RV dilation present | Heart failure exacerbation 47 days after RHC date |
| Subject 4 | 50 y.o. female, African American | Hypertension, heart failure, asthma, OSA | CKD5 on dialysis for 7 years via AVF | Sildenafil 30 mg every 8 h |
Combined PH Severe PH |
mPAP 47, PCWP 24, PVR 5, CO 4.6, CI 2.5 | None | – | LVEF 65%. No DD. ePASP ~45 mmHg. TRV 3.21. RV strain and dilation present | Died 284 days after RHC date |
| Subject 5 | 68 y.o. male, African American | Hypertension, diabetes mellitus, COPD, prostate cancer | CKD5 on dialysis for 2 years via AVF | Sildenafil 20 mg every 8 h, nifedipine 30 mg every 12 h |
Precapillary PH Non‐severe PH |
mPAP 27, PCWP 4, PVR 5.1, CO 4.5. CI 2.3 | None | – | LVEF 35%. DD present. ePASP ~40 mmHg. TRV 1.84. RV strain and dilation present | Died 58 days after RHC date |
| Subject 6 | 62 y.o. male, African American | Hypertension, diabetes mellitus, heart failure, COPD, | CKD5 on dialysis for 10 years via AVF | Sildenafil 20 mg every 8 h, amlodipine 10 mg daily |
Precapillary PH Severe PH |
mPAP 32, PCWP 6, PVR 8.4, CO 3.1, CI 1.9 | None | ‐ | LVEF 55%. DD present. ePASP ~75 mmHg. TRV 4.31. RA dilation present | None |
| Subject 7 | 51 y.o. male, African American | Liver cirrhosis | CKD5 on dialysis for 11 years via AVF | Ambrisentan 10 mg daily |
Combined PH Severe PH |
mPAP 50, PCWP 32, PVR 3.5, CO 5.1, CI 2.9 | mPAP 19, PCWP 7, PVR 1.9, CO 6.2, CI 3.5 | 7 months | LVEF 55%. DD present. ePASP ~55 mmHg. TRV 3.16. RV dilation present | None |
| Subject 8 | 58 y.o. female, African American | Hypertension, diabetes mellitus, heart failure | CKD5 not on dialysis. Baseline Cr 3.7 and eGFR 13 | Sildenafil 20 mg every 8 h |
Precapillary PH Severe PH |
mPAP 35, PCWP 13, PVR 3.2, CO 6.9, CI 3.6 | None | – | LVEF 45%. DD present. ePASP ~68 mmHg. TRV 3.61. RV strain and dilation present | Heart failure exacerbation 261 days after RHC date |
| Subject 9 | 61 y.o. male, African American | Hypertension, COPD | CKD5 on dialysis for 6 years via AVF | Sildenafil 20 mg every 8 h, amlodipine 5 mg daily |
Precapillary PH Non‐severe PH |
mPAP 33, PCWP 5, PVR 4.7, CO 5.9, CI 3.4 | None | – | LVEF 55%. DD present. ePASP ~70 mmHg. TRV 3.35. RA dilation present | None |
| Subject 10 | 59 y.o. male, African American | Hypertension, diabetes mellitus, OSA | CKD5 on dialysis for 3 years via AVF | Sildenafil 10 mg every 8 h, nifedipine 30 mg every 12 h |
Precapillary PH Severe PH |
mPAP 38, PCWP 5, PVR 5.2, CO 6.3, CI 3.3 | None | – | LVEF 55%. DD present. ePASP ~60 mmHg. TRV 3.59. RV strain and dilation present | None |
| Subject 11 | 73 y.o. female, Caucasian | Hypertension, diabetes mellitus, heart failure, ILD | CKD4 not on dialysis. Baseline Cr 2.18 and eGFR 24 | Sildenafil 20 mg every 8 h |
Combined PH Severe PH |
mPAP 55, PCWP 36, PVR 5.5, CO 3.5, CI 2 | None | – | LVEF 70%. No DD. ePASP ~62 mmHg. TRV 3.83. RV strain and dilation present | None |
| Subject 12 | 69 y.o. male, African American | Hypertension, diabetes mellitus, liver cirrhosis, heart failure, | CKD4 not on dialysis. Baseline Cr 2.7 and eGFR 23 | Sildenafil 20 mg every 8 h |
Combined PH Severe PH |
mPAP 45, PCWP 23, TPG 22, PVR 2.7, CO 8.2, CI 4.1 | None | – | LVEF 55%. DD not assessed. ePASP ~90 mmHg. TRV 3.68. RV strain and dilation present | None |
| Subject 13 | 61 y.o. female, Caucasian | Hypertension, diabetes mellitus, heart failure | CKD4 not on dialysis. Baseline Ct 1.83 and eGFR 28 | Sildenafil 40 mg every 8 h, amlodipine 5 mg daily |
Combined PH Severe PH |
mPAP 36, PCWP 20, PVR 3.4, CO 4.8, CI 2.5 | mPAP 29, PCWP 13, PVR 2.4, CO 6.6, CI 3.4 | 10 months | LVEF 65%. DD not assessed. ePASP ~45 mmHg. TRV 2.73. RV strain and dilation present | Heart failure exacerbation 79 days after RHC date |
| Subject 14 | 74 y.o. female, Asian | Hypertension, liver cirrhosis, heart failure | CKD5 on dialysis for 3 years via AVF | Sildenafil 20 mg every 8 h, nifedipine 60 mg daily |
Precapillary PH Severe PH |
mPAP 49, PCWP 11, PVR 5.5, CO 7, CI 5 | mPAP 28, PCWP 6, PVR 4.6, CO 4.8, CI 3.4 | 2 months | LVEF 55%. DD present. ePASP ~70 mmHg. TRV 3.33. RV dilation present | None |
| Subject 15 | 59 y.o. male, African American | Hypertension, heart failure | CKD5 not on dialysis. Baseline Cr 5.3 and eGFR 12 | Tadalafil 20 mg |
Combined PH Severe PH |
mPAP 47, PCWP 30, TPG 17, PVR 1.9, CO 9, CI 4.4 | None | – | LVEF 35%. No DD. ePASP ~40 mmHg. TRV 2.19. RV strain and dilation present | None |
| Subject 16 | 75 y.o. female, Caucasian | Heart failure | CKD5 not on dialysis. Baseline Cr 3.3 and eGFR 14 | Sildenafil 20 mg every 8 h |
Precapillary PH Non‐severe PH |
mPAP 34, PCWP 6, PVR 6.8, CO 4.1, CI 2.1 | None | – | LVEF 35%. DD not assessed. ePASP ~52 mmHg. TRV 3.12. RV strain and dilation present | None |
| Subject 17 | 58 y.o. female, African American | Hypertension | CKD5 on dialysis for 4 years via AVF | Sildenafil 10 mg every 8 h, amlodipine 5 mg daily |
Combined PH Severe PH |
mPAP 52, PCWP 23, PVR 4.8, CO 6, CI 3 | None | – | LVEF 55%. DD not assessed. ePASP ~45 mmHg. TRV 3.15. RA dilation present | None |
Abbreviations: CI, cardiac index; CKD, chronic kidney disease; CO, cardiac output; COPD, chronic obstructive pulmonary disease; DD, diastolic dysfunction; eGFR, estimated glomerular filtration rate; ePASP, estimated pulmonary artery systolic pressure; ILD, interstitial lung disease; LA, left atrium; LVEF, left ventricular ejection fraction; mPAP, mean pulmonary artery pressure; mRAP, mean right atrial pressure; OSA, obstructive sleep apnea; PCWP, pulmonary capillary wedge pressure; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; RHC, right heart catheterization; TPG, transpulmonary gradient; TRV, tricuspid regurgitation velocity.
In terms of treatments, 94% (n = 16) of patients received phosphodiesterase‐5 (PDE5) antagonists, mainly sildenafil (n = 15), except one who received tadalafil. Two patients (12%) received the endothelin receptor antagonist (ERA) ambrisentan, one in combination with sildenafil and one as a single agent. Forty‐seven percent (n = 8) of patients were simultaneously on calcium channel blocker (CCB) therapy. Their individualized dosage and frequency are presented in Table 2.
Within a year after RHC, 35% (n = 6) of patients had adverse clinical outcomes; 4/6 with isolated precapillary‐PH, and 2/6 with combined‐PH. 4/6 developed CV‐related outcomes (mainly heart failure [HF] exacerbation), whereas 3/6 died within a year. 5/6 of these patients already had severe PH at baseline.
Only 4 patients had repeated hemodynamic assessments within a median of 5 months (range 2–10); three had improved hemodynamics (decreased mPAP and PVR), whereas one patient (subject 1) had worsened hemodynamics (increased mPAP and PVR and decreased CI) (Figure 1A,B). Of the three subjects with improved hemodynamics, one was admitted to the Hospital for HF exacerbation 77 days after the index RHC. The only subject with worsened hemodynamics was non‐adherent to medications, developed a stroke, and died 222 days after the RHC date.
FIGURE 1.
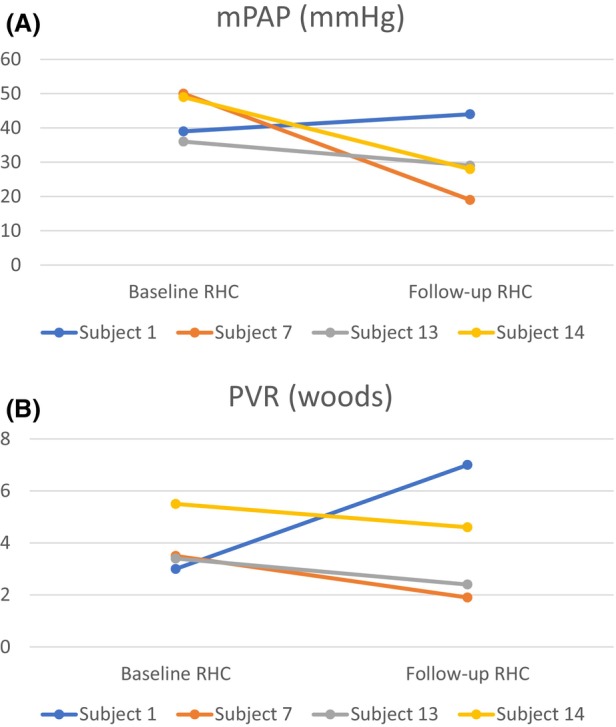
(A, B). Line plots showing change in hemodynamic parameters at baseline and repeated RHC.
DISCUSSION
We described a case series of 17 patients with CKD stages 4 or 5 and precapillary‐PH or combined‐PH confirmed by RHC treated with pulmonary vasodilators. Although the use of pulmonary vasodilator therapy is a potential therapeutic option for CKD patients with precapillary PH, the safety and efficacy of these medications have yet to be established in these patients and tend to be underutilized. Indeed, in our study population, only 6% of patients with a precapillary component of PH were treated with pulmonary vasodilators.
In the present study, despite the use of vasodilator therapy, more than one‐third (35%) of patients still developed adverse clinical outcomes. Certainly, several possible reasons exist for the elevated morbidity and mortality among PH‐CKD patients treated with pulmonary vasodilators.
First, patients who developed adverse clinical outcomes were sick at baseline, including 5/6 patients with severe PH and 4/6 with end‐stage kidney disease requiring dialysis. The median one‐year survival time of dialysis patients is about 80%, and similar survival rates have been reported among PH‐CKD patients. 3 , 17 Therefore, adverse outcomes in PH‐CKD patients are expected even if pulmonary vasodilator therapy does offer some benefit.
Second, HF with preserved ejection fraction (HFpEF) is prevalent among CKD patients, and pulmonary vasodilators are likely to trigger HF decompensation in these patients. Although 2/3 episodes of HF exacerbations occurred among patients with isolated precapillary‐PH, both had diastolic dysfunction on TTE suggestive of occult HFpEF. This represents a major limitation that must be acknowledge in future potential trials of pulmonary vasodilators in this population. Of note, PDE‐5 antagonists, the most used vasodilators in our study, have been associated with encouraging outcomes in patients with combined‐PH owing to their ability to improve LV relaxation in addition to vasodilating the pulmonary vessels. 18
Third, although 3/4 of patients showed improved hemodynamics upon repeated RHC, one subject still developed HF exacerbation and required hospitalization. Indeed, better pulmonary hemodynamics (lower mPAP, PVR, and higher CI) do not necessarily translate into clinical benefit in PH‐CKD patients. For instance, comorbid conditions such as chronic lung diseases, present in 35% of our patients, may worsen with the use of systemic pulmonary vasodilators. 19
Our study had several limitations, including its single‐center and retrospective design. We included only a small number of patients who met the study criteria, which was expected as patients with PH‐CKD usually do not receive pulmonary vasodilator therapy. We used RHC hemodynamic assessment to classify their hemodynamic phenotype (isolated precapillary‐PH vs. combined‐PH). We recognize this is only a snapshot of their underlying hemodynamic and prone to misclassification. Hence, we documented diastolic dysfunction on TTE as a surrogate of occult HFpEF. Due to the retrospective nature of the study, we could not ascertain whether the simultaneous use of other medications or poor treatment adherence also impacted our patients' outcomes. Future studies of vasodilator therapy in PH‐CKD patients must be prospective, use a strict selection criterion (excluding patients with combined‐PH and occult HFpEF), and use a control group as comparison. Future research will likely require looking at surrogate outcomes similar to PAH studies, such as improvement in exercise capacity, impact in quality of life, or measurement of clinically relevant biomarkers. 20
In summary, among advanced CKD patients with precapillary or combined PH, the use of pulmonary vasodilators was uncommon and did not prevent adverse clinical outcomes within a year of follow‐up. Given the high prevalence of CKD patients with a precapillary component of PH, further research into the use of pulmonary vasodilators in this specific subset of patients is warranted.
AUTHOR CONTRIBUTIONS
Project conceptualization and design: Raul Leguizamon, Ian McLaren, Jose Manuel Martinez Manzano. Acquisition, analysis and interpretation of data: Raul Leguizamon, Ian McLaren, Tara John, Rasha Khan, Alexander Prendergast, Phuuwadith Wattanachayakul, Andrew Geller, John Malin, Simone A. Jarret, Christian Witzke, Kevin Bryan Lo, Jose Manuel Martinez Manzano. Manuscript writing – Original draft: Raul Leguizamon, Ian McLaren, Jose Manuel Martinez Manzano. Critical revision of the manuscript for intellectual content: Raul Leguizamon, Ian McLaren, Tara John, Rasha Khan, Alexander Prendergast, Phuuwadith Wattanachayakul, Andrew Geller, John Malin, Simone A. Jarret, Christian Witzke, Kevin Bryan Lo, Jose Manuel Martinez Manzano. Statistical analysis: Jose Manuel Martinez Manzano.
FUNDING INFORMATION
No funding was required for this study.
CONFLICT OF INTEREST STATEMENT
Given the adherence to ethical and institutional guidelines and the main incentive of academic growth without monetary incentive for the development of this study, the authors declare no conflicts of interest present in this study.
ETHICS STATEMENT
This study was approved by the Jefferson Institutional Review Board (iRISID‐2023‐2277).
Leguizamon R, McLaren I, John T, Khan R, Prendergast A, Wattanachayakul P, et al. Pulmonary vasodilators in patients with advanced chronic kidney disease and pre‐capillary pulmonary hypertension—A case series. Respirology Case Reports. 2024;12(9):e70027. 10.1002/rcr2.70027
Raul Leguizamon and Ian McLaren have contributed equally to this work.
Associate Editor: Trevor Williams
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Bolignano D, Rastelli S, Agarwal R, Fliser D, Massy Z, Ortiz A, et al. Pulmonary hypertension in CKD. Am J Kidney Dis. 2013;61(4):612–622. 10.1053/j.ajkd.2012.07.029 [DOI] [PubMed] [Google Scholar]
- 2. Agarwal R. Prevalence, determinants and prognosis of pulmonary hypertension among hemodialysis patients. Nephrol Dial Transplant. 2012;27(10):3908–3914. 10.1093/ndt/gfr661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Edmonston DL, Parikh KS, Rajagopal S, Shaw LK, Abraham D, Grabner A, et al. Pulmonary hypertension subtypes and mortality in CKD. Am J Kidney Dis. 2020;75(5):713–724. 10.1053/j.ajkd.2019.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zeder K, Siew ED, Kovacs G, Brittain EL, Maron BA. Pulmonary hypertension and chronic kidney disease: prevalence, pathophysiology and outcomes. Nat Rev Nephrol. 2024. 10.1038/s41581-024-00857-7 [DOI] [PubMed] [Google Scholar]
- 5. Belém LC, Zanetti G, Souza AS, et al. Metastatic pulmonary calcification: state‐of‐the‐art review focused on imaging findings. Respir Med. 2014;108(5):668–676. 10.1016/j.rmed.2014.01.012 [DOI] [PubMed] [Google Scholar]
- 6. Roumeliotis S, Mallamaci F, Zoccali C. Endothelial dysfunction in chronic kidney disease, from biology to clinical outcomes: a 2020 update. J Clin Med. 2020;9(8):2359. 10.3390/jcm9082359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Malin J, Khan R, Manzano JMM, et al. Association of arteriovenous fistulae with precapillary pulmonary hypertension—a single center retrospective analysis of invasive hemodynamic parameters. Hear Lung J Cardiopulm Acute Care. 2024;68:260–264. 10.1016/j.hrtlng.2024.08.007 [DOI] [PubMed] [Google Scholar]
- 8. O'Leary JM, Assad TR, Xu M, Birdwell KA, Farber‐Eger E, Wells QS, et al. Pulmonary hypertension in patients with chronic kidney disease: invasive hemodynamic etiology and outcomes. Pulm Circ. 2017;7(3):674–683. 10.1177/2045893217716108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arevalo C, White RJ, Le T, Lachant D. Vasodilator use in precapillary pulmonary hypertension with end stage kidney disease: a single center experience. Respir Med. 2021;188:106596. 10.1016/j.rmed.2021.106596 [DOI] [PubMed] [Google Scholar]
- 10. Kimuro K, Hosokawa K, Abe K, et al. Beneficial effects of pulmonary vasodilators on pre‐capillary pulmonary hypertension in patients with chronic kidney disease on hemodialysis. Life. 2022;12(6):780. 10.3390/life12060780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Umetani K, Atsumi M. Haemodialysis patient with chronic kidney disease and pulmonary hypertension treated effectively with pulmonary vasodilators. BMJ Case Rep. 2023;16(12):e255810. 10.1136/bcr-2023-255810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Colombijn JMT, Idema DL, van Beem S, Blokland AM, van der Braak K, Handoko ML, et al. Representation of patients with chronic kidney disease in clinical trials of cardiovascular disease medications: a systematic review. JAMA Netw Open. 2024;7(3):e240427. 10.1001/jamanetworkopen.2024.0427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–830. 10.7326/0003-4819-158-11-201306040-00007 [DOI] [PubMed] [Google Scholar]
- 14. Galiè N, McLaughlin VV, Rubin LJ, Simonneau G. An overview of the 6th world symposium on pulmonary hypertension. Eur Respir J. 2019;53(1):1802148. 10.1183/13993003.02148-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):1801913. 10.1183/13993003.01913-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brewis MJ, Church AC, Johnson MK, Peacock AJ. Severe pulmonary hypertension in lung disease: phenotypes and response to treatment. Eur Respir J. 2015;46(5):1378–1389. 10.1183/13993003.02307-2014 [DOI] [PubMed] [Google Scholar]
- 17. Ferreira E d S, Moreira TR, da Silva RG, et al. Survival and analysis of predictors of mortality in patients undergoing replacement renal therapy: a 20‐year cohort. BMC Nephrol. 2020;21(1):502. 10.1186/s12882-020-02135-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ovchinnikov A, Potekhina A, Belyavskiy E, Ageev F. Heart failure with preserved ejection fraction and pulmonary hypertension: focus on phosphodiesterase inhibitors. Pharmaceuticals. 2022;15(8):1024. 10.3390/ph15081024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nathan SD, Barbera JA, Gaine SP, Harari S, Martinez FJ, Olschewski H, et al. Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J. 2019;53(1):1801914. 10.1183/13993003.01914-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sitbon O, Gomberg‐Maitland M, Granton J, Lewis MI, Mathai SC, Rainisio M, et al. Clinical trial design and new therapies for pulmonary arterial hypertension. Eur Respir J. 2019;53(1):1801908. 10.1183/13993003.01908-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


