Figure 1.
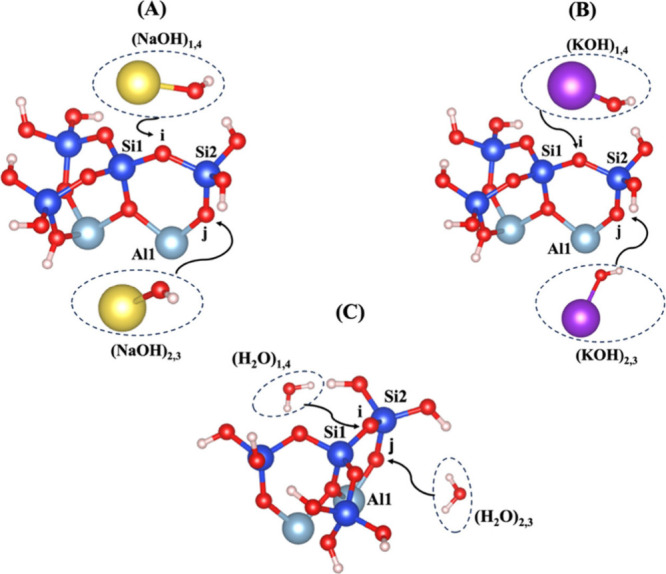
Illustration of different scenarios, resulting in dissolution of silicate tetrahedra (SiO44–) through (A) sodium hydroxide (NaOH), (B) potassium hydroxide (KOH), or (C) water (H2O), depending on the bridging oxygens bonded to the silicate and aluminate neighboring units. Silicon ions are shown in blue; aluminum cations, in light blue; oxygen ions, in red; hydrogen protons, in white; sodium cations, in yellow; potassium cations, in purple.
