Fig. 6. Local and systemic clearance of contrast agents in different solutions.
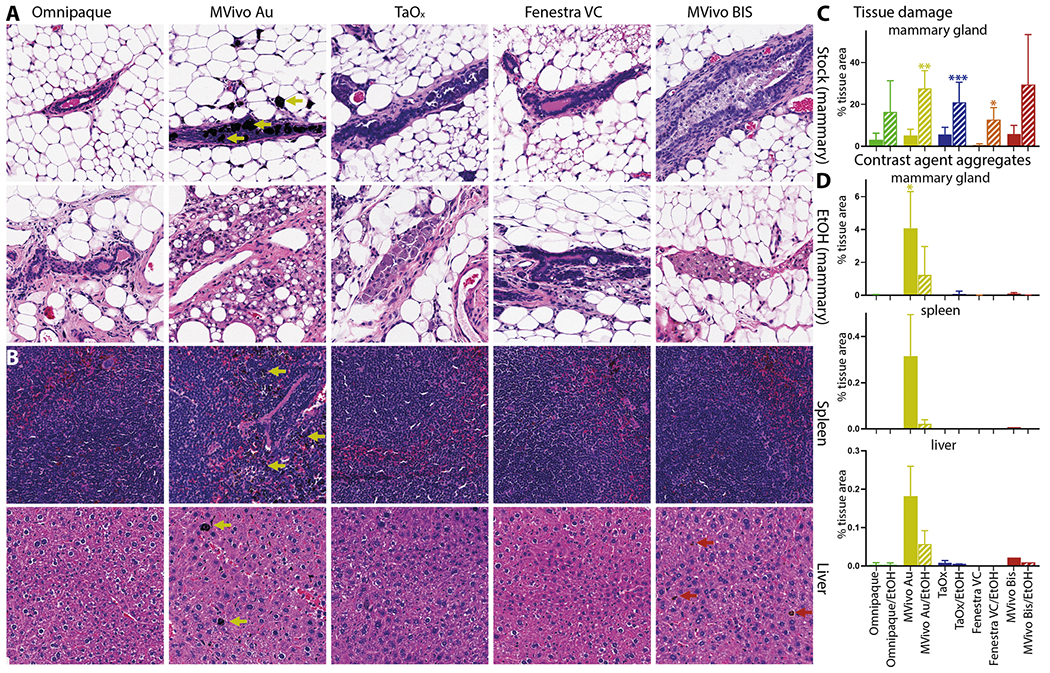
Representative H&E staining of the mammary gland, spleen, and liver 60 days after ID injection of indicated contrast agent as stock solution (A, B): Omnipaque (300 mg I/ml), MVivo Au (200 mg Au/ml), TaOx (36 mg Ta/ml), Fenestra VC (50 mg I/ml), MVivo BIS (150 mg Bis/ml), or in 70% ethanol (EtOH) (A): Omnipaque (90 mg I/ml), MVivo Au (60 mg Au/ml), TaOx (10.8 mg Ta/ml), Fenestra VC (15 mg I/ml), MVivo BIS (45 mg Bis/ml). Arrows point to nanoparticle aggregates. C Morphology-driven quantitation of tissue damage, which includes fibrosis, inflammation and scarring resulting from ablative effects of 70% EtOH as well as immune cell-mediated foreign object reaction to clear nanoparticle-based contrast agents. D Quantitation of visually apparent aggregates of nanoparticle-based contrast agents in indicated tissues. Asterisks indicate p value of unpaired Welch’s t-test of each solution compared to Omnipaque stock (* < 0.01, ** <0.001, *** <0.0001).
