Figure 2.
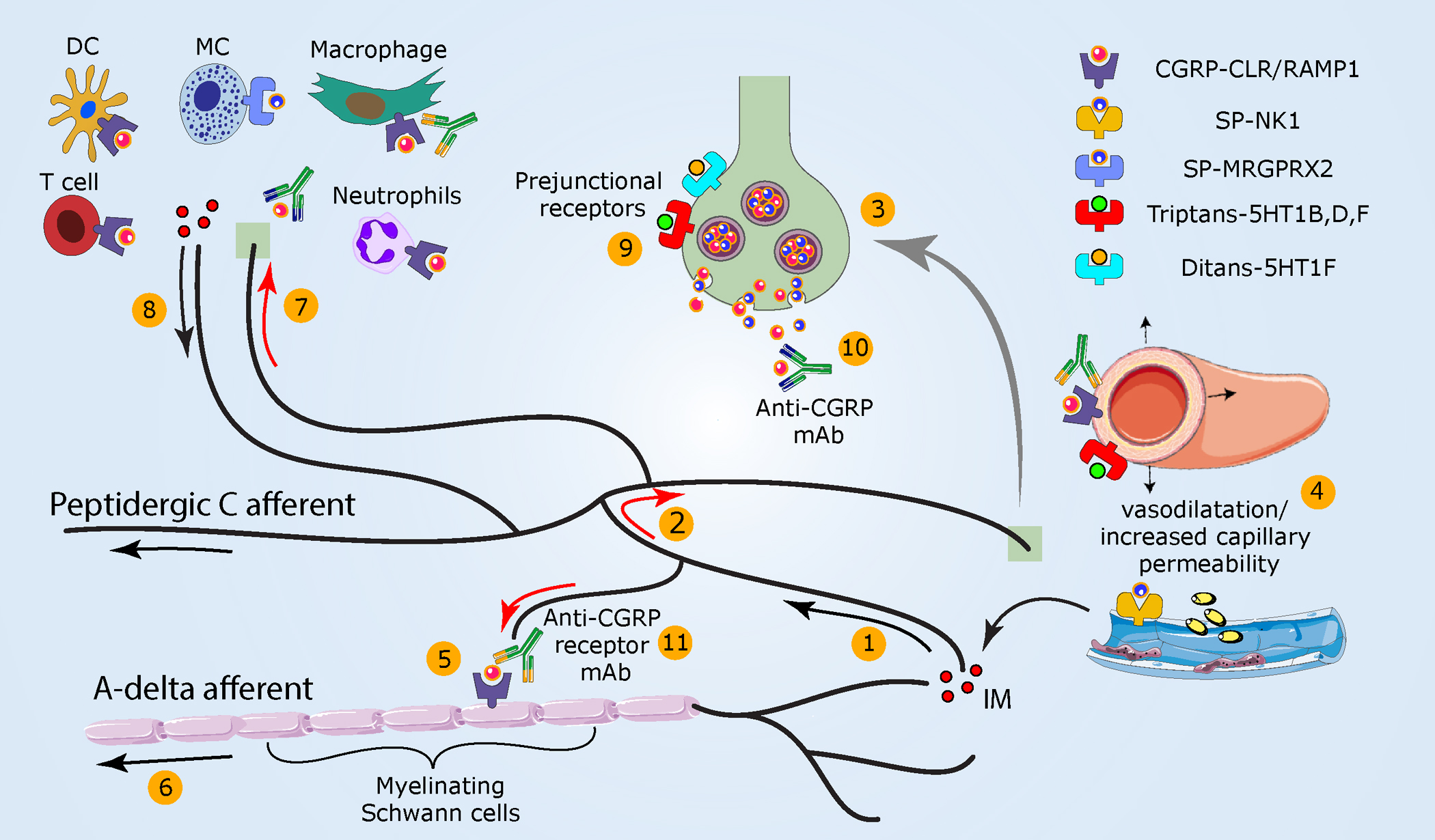
Meningeal neurogenic inflammation as a potential migraine headache mechanism. (①) As noted in Figure 1, the activation of peptidergic meningeal afferents (②) generates an antidromic axonal reflex (red arrows) and (③) neuropeptide release. (④) Subsequently, vasodilatation, increased capillary permeability, and IM release ensue. (⑤) CGRP, locally released, can also engage cell surface receptors that internalize on Schwann cells ensheathing myelinated A-delta meningeal afferents. Hypothesized downstream mechanisms include persistent CGRP receptor signaling within endosomes that (⑥) enhances A-delta afferent activity via TRPA1-dependent mechanisms. (⑦) Released sensory neuropeptides may also drive a local meningeal neurogenic immune response (⑧) that could further sensitize meningeal afferents. (⑨) Triptans used to abort migraine headache activate prejunctional 5-HT1B/1D/1F receptors on meningeal afferent terminals to inhibit the release of neuropeptides and related development of meningeal neurogenic inflammation. Triptans additionally act on vascular receptors to produce arterial vasoconstriction. Activation of prejunctional 5-HT1F receptors inhibits meningeal afferent neuropeptide release and aborts migraine pain without causing vasoconstriction. CGRP receptor antagonists block meningeal neurogenic vasodilatation and abort migraine headaches. (⑩) mAbs that bind CGRP or its receptor are used preventatively in the clinic and act selectively in the periphery. Abbreviations: CGRP, calcitonin gene–related peptide; CLR, calcitonin receptor–like receptor, IM, inflammatory mediator; mAb, monoclonal antibody; MRGPRX2, Mas-related G protein–coupled receptor-X2; NK1, neurokinin 1 receptor; RAMP1, receptor activity–modifying protein-1; SP, substance P.
