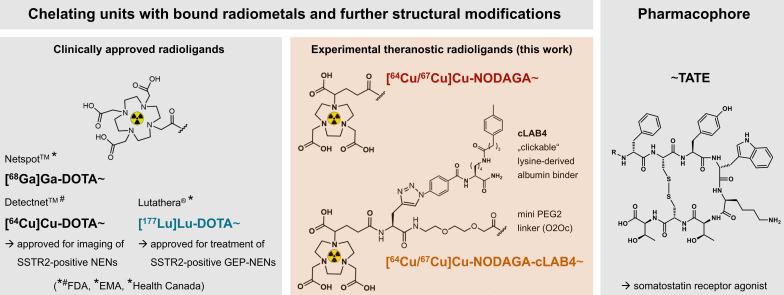
Figure 1.
Clinically approved TATE variants for theranostic application in the management of SSTR2-positive neuroendocrine neoplasms and experimental variants (this work) for theranostic use of radiocopper; pharmacokinetic properties of the diagnostic variants [64Cu]Cu‑NODAGA-TATE and [64Cu]Cu‑NODAGA‑cLAB4-TATE in tumor-bearing mice have been reported previously 24; (cLAB4) 'clickable' lysine-derived albumin binder 4; (EMA) European Medicines Agency; (FDA) Food and Drug Administration of the United States; (GEP) gastroenteropancreatic; (NENs) neuroendocrine neoplasms.