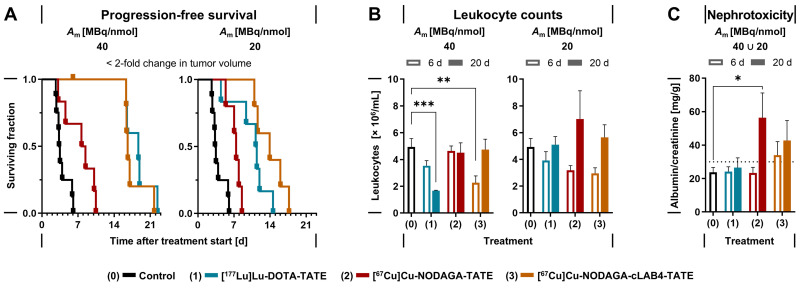
Figure 4.
Treatment effects of lutetium‑177- and copper‑67-labeled TATE variants in MPC allograft mice; all animals received an initial activity dose of 50 MBq corresponding to molar amounts of 1.25 nmol (Am = 20 MBq/nmol) or 2.5 nmol (Am = 20 MBq/nmol) at treatment start; (A) Progression-free survival (PFS) defined as < 2-fold change in tumor volume compared to treatment start; censored data points along horizontal lines indicate animals withdrawn from follow-up before exceeding the PFS threshold; (B) Leukocyte counts in blood at indicated time points after treatment start; (C) Albumin/creatinine ratio (ACR) in urine estimating nephrotoxicity at indicated time points after treatment start, pooled data from treatments at different molar activities; (dotted line) lower threshold for microalbuminuria at ACR > 30 < 300 mg/g; data presented as means ± standard error; significance of differences: * p < 0.05, ** p < 0.01, *** p < 0.001.