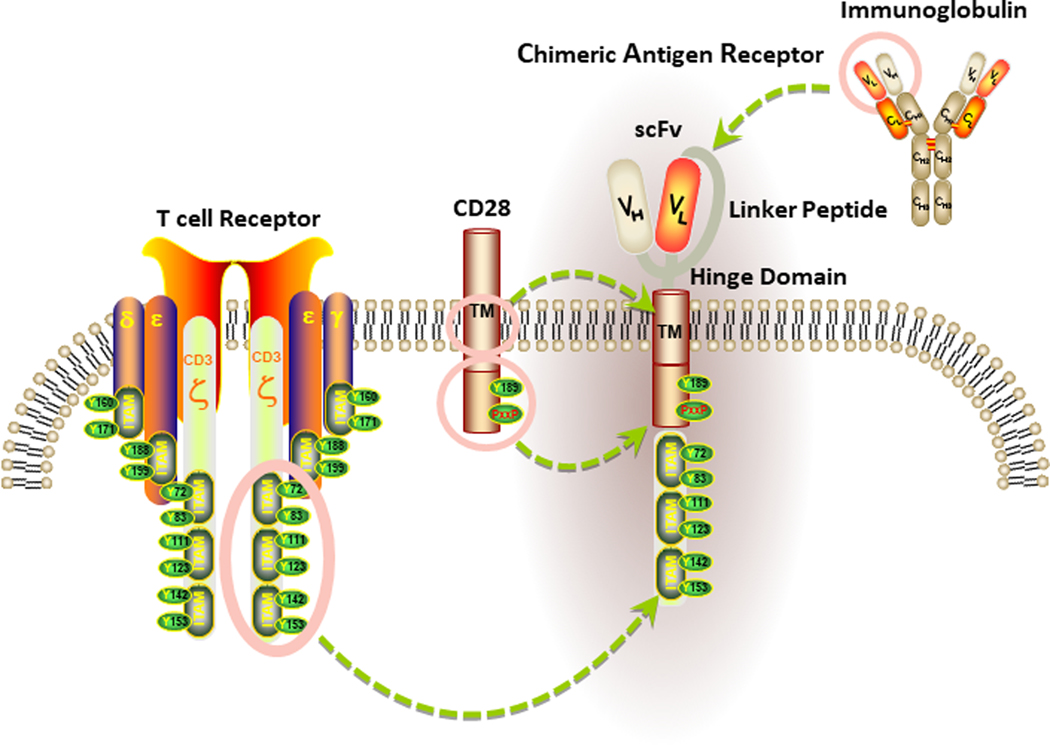
Figure 1. Basic Structure of a chimeric antigen receptor (CAR).
The antigen recognition domain of a CAR is typically a single-chain variable fragment (scFv) comprised of the variable light (VL) and heavy (VH) chains of an immunoglobulin, connected by a short linker peptide. This binding moiety is fused to a hinge region that is anchored to the plasma membrane by a transmembrane (TM) domain. In the diagram above, the TM domain of the CAR is derived from the CD28 costimulatory receptor. Signaling components of a CAR are localized within the receptor endodomain. Because endogenous T cell activation requires the phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs), the cytoplasmic portion of CD3ζ is commonly used as the main endodomain component of a CAR to drive signal 1. Signal 2, which is provided in the form of costimulation and is required for optimal T cell activation, is triggered by activation of an intracellular costimulatory receptor endodomain fused to CD3ζ (e.g., CD28).