SUMMARY
The purpose of this study was to examine the possible association between cheilitis and allergic reactions, and to use allergy skin tests to identify the allergens that induce allergic reactions in cheilitis patients (type I and type IV). We included 50 patients with recurrent cheilitis (reversible cheilitis) who were dermatologically examined and agreed to undergo allergy skin tests, i.e., patch test and prick test. Additionally, clinical pictures and patient mental stress levels were examined using the Perceived Stress Scale (PSS). Positive prick tests (atopy) were recorded in 84% of patients with cheilitis. The most frequently found allergens were contact allergens (54%) (cobalt chloride, nickel sulfate and thimerosal) and inhalant allergens (46%). The patch test positive subjects who used cosmetic, hygiene, and decorative products were significantly more likely to have swollen and red lips than the patch test negative subjects. Also, low stress levels were recorded less frequently in patients with confirmed allergies than in non-allergic patients. The results indicated a higher incidence of cheilitis in the people prone to allergies (atopics) and confirmed an association between cheilitis and allergies. To our knowledge, this is the first study in patients with cheilitis, which simultaneously analyzed allergies, their clinical features and PSS in the same patients.
Key words: Cheilitis, Lip inflammation, Allergy, Allergens, Mental stress
Introduction
Allergic reactions can cause inflammation of the lips, with possible type I allergic reaction, i.e., immediate IgE-mediated allergic reaction, or delayed allergic hypersensitivity (type IV) to some substance. It has been proven that people with atopy (those with elevated IgE values who are prone to allergies of the immediate type, type I) are predisposed to cheilitis simplex, exfoliative cheilitis, angular cheilitis and glandular cheilitis (1-7). According to Hanifin and Rajka, the authors of the valid criteria for diagnosing atopic dermatitis (AD), the presence of lip inflammation (cheilitis) is one of the minor criteria for diagnosing AD (8, 9). Also, in the classification of cheilitis, some authors mention atopic cheilitis as a special form of cheilitis within AD (1, 3). In addition to immediate reactions, there is a delayed type of allergic reactions, i.e., type IV (cellular hypersensitivity), causing contact allergic cheilitis (cheilitis venenata, according to some authors) which is, for instance, mentioned in the etiopathogenesis of exfoliative cheilitis (1, 4, 10). Also, it is estimated that 22% of angular cheilitides is caused by a contact allergic reaction to the materials from which orthodontic appliances are made (1, 11).
The possible allergens as etiologic factors for cheilitis are cosmetic, hygienic and decorative products (toothpastes, balms and lipsticks, creams, nail polishes, hair sprays), dental materials, latex, metals for cutlery and wind instruments, some foodstuffs (nuts, curry, cinnamon, mushrooms, citrus, mango, pineapple, parsley, etc.), preservatives, medicines (e.g., acyclovir) and other objects that come in contact with the lips (1, 10, 12, 13). However, in most cases, contact allergic cheilitis is caused by a reaction to balms and lipsticks, which is why the term lipstick cheilitis can be found in the literature (5, 12). Particularly important contact allergens relevant for the occurrence of cheilitis are metals; for example, allergy to nickel in persons wearing orthodontic appliances can manifest as angular cheilitis (1). Dental materials that can cause an allergic reaction include mercury, cobalt, chromium, impression materials, eugenol, etc. (1, 13). Also, in patients with granulomatous cheilitis, allergy to cinnamon and benzoates has been shown to be a possible predisposing factor for the disease (10, 14).
In addition, for some types of cheilitis, psychological stress is mentioned as a possible related factor or trigger. Emotional, i.e., psychological stress can be the cause of exfoliative cheilitis, since people under psychological stress are more prone to undesirable habits that can lead to lip lesions (15). Stress is also mentioned in the etiopathogenesis of factitious cheilitis, in which self-harm due to stress and psychiatric disorders play etiologic roles, which is important for clinical practice as this cheilitis may resemble exfoliative cheilitis (16). In addition to stress, mental disorders also are the possible predisposing factors for the appearance of angular heilitis (e.g., trauma in bulimics) (10). Psychological stress is also a factor that can trigger or worsen allergic diseases such as AD, including its manifestations in the form of inflammatory lip lesions (9).
Therefore, the purpose of this study was to examine the possible association between allergic reactions and lesions on the lips that manifest as cheilitis, and to examine and identify the allergens that induce type I (prick test) and type IV (patch test) allergic reactions manifesting as cheilitis, with the aim of contributing to better identification of the etiopathogenic factors, as well as better and timely treatment that includes removal of allergens if identified.
Subjects and Methods
Patients were included in the study over a 15-month period (January 2019-March 2020); these were patients with lip lesions who came to the Department of Dermatovenereology, Sestre milosrdnice University Hospital Center and School of Dental Medicine because of inflammatory lesions of the lips or for some other reason. Their participation was voluntary and they had to sign an informed consent form. The research was carried out in accordance with the basic principles of the Declaration of Helsinki and was approved by the Ethics Committee of the Sestre milosrdnice University Hospital Center (approval no.: EP-520/19-8) and Ethics Committee of the School of Dental Medicine, University of Zagreb (approval no.: 05-PA -30-XI-11/2019).
The inclusion criteria for participation in the study were adult patients (>18 years of age) with inflammatory lip changes/lesions manifesting as reversible/recurrent cheilitis, who were examined by a dermatologist from our research team. The excluding factors were patients diagnosed with herpetic cheilitis, drug-induced cheilitis, irreversible form of cheilitis (actinic, granulomatous, glandular, plasma cell) and cheilitides associated with dermatoses and systemic diseases (pemphigus, lichen and angioedema, etc.) (10). Other excluding factors were lip lesions of another nature, e.g., developmental lesions, trauma, vascular and pigmented lesions, malignant lesions, other non-inflammatory lesions of benign nature, mucocele, etc.
So, among the initially examined 130 subjects with different types of cheilitis, 94 patients were diagnosed with reversible cheilitis. The patients diagnosed with reversible cheilitis were offered to undergo allergy skin tests, to which 50 patients agreed. Finally, the study included 50 patients who underwent two allergy tests, i.e., patch test (PT) for contact allergens and standard prick test (SPT) for inhalant and food allergens, as well as preservatives and additives.
First, all 50 patients with cheilitis were clinically examined by a dermatovenereologist. Localization of lesions (upper lip, lower lip, vermilion border, oral angles) and clinical forms of lesions/changes (erythema, dryness, erosions, fissures, ulcerations, hyperkeratosis, desquamation, plaques, purulent exudate, serous exudate, crusts, papules, vesicles, edema, hardening, erythema multiforme, lichenoid reaction) were recorded/identified.
Allergy skin tests
Patch test for contact allergens is an epicutaneous test that examines the delayed type of allergic reaction (type IV). It was performed using the commercial contact allergens (standard series) of the Institute of Immunology Zagreb, Croatia (17, 18). The subjects were also tested with additional dental allergens, i.e., methyl methacrylate [2.0% petrolatum (pet.), 2-hydroxyethyl methacrylate (2-HEMA) (2.0% pet.), ethylene glycol dimethacrylate (2.0% pet.) and triethylene glycol dimethacrylate (2.0% pet.)]. The test was performed by applying an allergen to a person’s upper back using Curatest adhesive tape (Lohmannand Rauscher, Germany). After 48 hours, 72 hours and 7 days of wearing the allergen, skin reactions/changes were read and entered according to the reading criteria. Positive findings are considered to be the results relevant to the International Contact Dermatitis Research Group (ICDRG) (17, 19).
Standard prick test was performed with standard commercial preparations (Diater Laboratorio, Spain) supplied by the Institute of Immunology, Zagreb, Croatia. Prick test was used to examine/determine inhalant allergens, i.e., mites Dermatophagoides pteronyssinus and Dermatophagoides farinae, cat epithelium, dog epithelium, pollen (grass, birch, hazel, cypress, olive, plane tree, poplar, ragweed (lemongrass), wormwood, dandelion), latex and mildew (Aspergillus fumigatus) (Diater Laboratorio, Spain). Prick test was also used for testing food allergens, i.e., cow’s milk, egg, gluten, wheat flour, cocoa, almonds, walnuts, peanuts, apples, oranges, bananas, tomatoes, beans, pork, beef, chicken, sardines, soy flour, sesame, hazelnut, strawberry, kiwi, watermelon, pineapple, peach, tuna, squid, shrimp, mussels (Diater Laboratorio, Spain). Preservatives and additives were also tested by the same method, including acetylsalicylic acid, sodium benzoate, tartazine, potassium metabisulfite, sodium glutamate, glutaraldehyde and citric acid (supplied by the Institute of Immunology, Zagreb, Croatia). A commercial histamine solution (1.0%) was used as positive control in the prick test, and a commercial buffer was used as negative control. Allergens were applied to the subject’s forearm and the readings were taken 15 minutes after application. If a wheal ≥3 mm in diameter occurred, with a negative buffer solution and positive reaction to histamine, it was considered a positive reaction to some allergen (19).
In further analysis, we examined the prevalence of allergic reactions and allergens recorded in patients with cheilitis who were tested. In addition, we wanted to examine whether lip lesions/symptoms occurring after using the potential contact allergens (cosmetic, hygiene and decorative products) were more common in patients with proven allergies to contact allergens than in those not allergic to contact allergens.
Perceived Stress Scale (PSS) determination
In patients with cheilitis, we also determined their level of psychological stress by determining their Perceived Stress Scale (PSS) values. All the participants filled in a questionnaire on the history connected to cheilitis, and their mental stress levels were examined using the PSS (20).
We also examined whether cheilitis patients with confirmed allergies had higher psychological stress than patients without confirmed allergies.
Analysis of the latest studies on cheilitis associated with allergies
We wanted to analyze clinical studies found in the Pubmed scientific database under the following key words: cheilitis, lip inflammation, allergy, allergens, allergy skin tests. The inclusion criterion was their publication in the last 5 years, that is, in the 2015-2020 period; we excluded case reports and reviews. We took into account the papers which had been published in the indexed medical publications and written in English.
Statistical analysis
On statistical analysis, the χ2-test for frequencies and z-test were used for comparison between individual groups with Bonferroni correction, Kruskal-Wallis test with Mann-Whitney post-hoc tests with Bonferroni corrections, and analysis of variance with Student-Newman-Keuls post-hoc test. The effect size was quantified by r and its equivalent Cramer V, τb, and η2. Cohen’s criteria r = 0.1-0.3 = low effect size, 0.3-0.5 = moderate, 0.5-0.7 = large, and >0.7 = very large were used for interpretation. Since the design of our study was observational-correlational on an appropriate/convenient sample, statistical power was analyzed/expressed as effect size for those statistical tests which were made. Also, for the analysis of parameters, we did not have a group for comparing their values with the values obtained in our patients, and the only way to carry out statistical analysis was to compare the results obtained with the expected probability ratio 50:50 and thus establish statistical significance of the results. IBM SPSS 22 commercial software (IBM Corp, Armonk, USA) was used.
Results
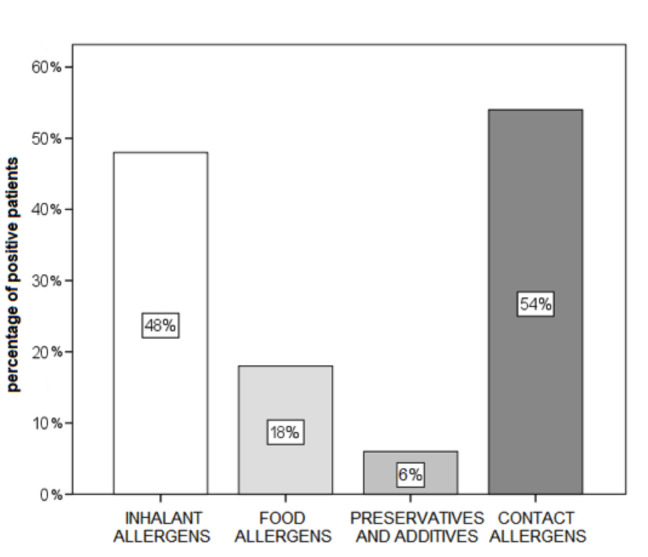
Of the total of 50 subjects tested, 84% were found to have atopy; we considered that atopics were those with a positive reaction to at least one allergen in the prick test. Concerning the results of allergy tests and the allergens obtained, positive reactions to contact allergens in the patch test turned out to be most frequent (54%), whereas positive reactions to preservatives and additives were least frequent (6%) (Fig. 1).
Fig. 1.
Proportion of subjects positive to at least one allergen in allergy skin tests (N=50).
The analysis of confirmed allergens showed that positive allergy tests were most commonly found for inhalant allergens (in the prick test) and for cobalt chloride, nickel sulfate, and thimerosal (in the patch test) (48%, 36%, 20% and 12%, respectively) (Table 1). Therefore, on statistical analysis, if we statistically expected that the same number of subjects were positive and negative for allergens (25 out of 50 subjects tested), differences between the expected and obtained values would be statistically significant for all except inhalant allergens (p≤0.048).
Table 1. Results of allergy skin tests in patients with cheilitis (N=50).
| Allergen | Negative | Positive | p | |
|---|---|---|---|---|
| Inhalant allergens – prick test | n (%) | 26 (52%) | 24 (48%) | 0.777 |
|
Food allergens – prick test Cow’s milk |
n (%) | 48 (96%) | 2 (4%) | <0.001 |
| Egg | n (%) | 49 (98%) | 1 (2%) | <0.001 |
| Gluten | n (%) | 49 (98%) | 1 (2%) | <0.001 |
| Wheat flour | n (%) | 47 (94%) | 3 (6%) | <0.001 |
| Cocoa | n (%) | 49 (98%) | 1 (2%) | <0.001 |
| Almond | n (%) | 49 (98%) | 1 (2%) | <0.001 |
| Walnut | n (%) | 48 (96%) | 2 (4%) | <0.001 |
| Peanut | n (%) | 49 (98%) | 1 (2%) | <0.001 |
| Banana | n (%) | 49 (98%) | 1 (2%) | <0.001 |
| Chicken | n (%) | 49 (98%) | 1 (2%) | <0.001 |
| Sardines | n (%) | 49 (98%) | 1 (2%) | <0.001 |
| Hazelnut | n (%) | 48 (96%) | 2 (4%) | <0.001 |
| Pineapple | n (%) | 47 (94%) | 3 (6%) | <0.001 |
| Peach | n (%) | 48 (96%) | 2 (4%) | <0.001 |
| Tuna | n (%) | 48 (96%) | 2 (4%) | <0.001 |
| Squid | n (%) | 49 (98%) | 1 (2%) | <0.001 |
| Shrimp | n (%) | 48 (96%) | 2 (4%) | <0.001 |
| Mussels | n (%) | 48 (96%) | 2 (4%) | <0.001 |
|
Preservatives and additives – prick test Sodium benzoate |
n (%) | 49 (98%) | 1(2%) | <0.001 |
| Glutaraldehyde | n (%) | 49 (98%) | 1 (2%) | <0.001 |
| Citric acid | n (%) | 49 (98%) | 1 (2%) | <0.001 |
|
Contact allergens – patch test Potassium dichromate |
n (%) | 48 (96%) | 2 (4%) | <0.001 |
| Cobalt chloride | n (%) | 32 (64%) | 18 (36%) | 0.048 |
| Nickel sulfate | n (%) | 40 (80%) | 10 (20%) | <0.001 |
| Balsam of Peru | n (%) | 48 (96%) | 2 (4%) | <0.001 |
| Colophony | n (%) | 49 (98%) | 1 (2%) | <0.001 |
| Ammoniated mercury | n (%) | 50 (100%) | 0 (0%) | <0.001 |
| Fragrance mix | n (%) | 49 (98%) | 1 (2%) | <0.001 |
| Thimerosal | n (%) | 44 (88%) | 6 (12%) | <0.001 |
| Lanolin | n (%) | 49 (98%) | 1 (2%) | <0.001 |
| Formaldehyde | n (%) | 49 (98%) | 1 (2%) | <0.001 |
When comparing the prevalence of cheilitis lesions/symptoms after using cosmetics, hygiene and decorative products in two groups of patients, we compared a group with at least one positive allergen in patch test with another group without proven allergens in patch test. The patch-test positive subjects who used cosmetic, hygiene and decorative products were significantly more likely to have swollen lips and red lips than patch-negative subjects, with moderate effect sizes (p=0.014 and p=0.008; r=0.356 and r=0.384, respectively) (Table 2).
Table 2. Comparison of the prevalence of cheilitis symptoms between the groups of subjects tested by patch test (N=48; 22 negative and 26 positive).
| Symptom | Patch test | AM | SD | p* |
|---|---|---|---|---|
| Lip itch | Negative | 0.2 | 0.6 | |
| Positive | 0.5 | 1.1 | 0.255 | |
| Lip burning | Negative | 0.4 | 0.7 | |
| Positive | 0.7 | 1.2 | 0.419 | |
| Lip fissures | Negative | 0.5 | 1.0 | |
| Positive | 0.9 | 1.2 | 0.190 | |
| Lip dryness | Negative | 0.6 | 1.1 | |
| Positive | 0.9 | 1.2 | 0.393 | |
| Lip desquamation | Negative | 0.5 | 0.9 | |
| Positive | 0.7 | 1.0 | 0.277 | |
| Lip edema | Negative | 0.2 | 0.6 | |
| Positive | 0.8 | 1.0 | 0.014 | |
| Lip erythema | Negative | 0.3 | 0.7 | |
| Positive | 1.2 | 1.3 | 0.008 | |
| Lip crusts | Negative | 0.2 | 0.6 | |
| Positive | 0.2 | 0.5 | 0.845 | |
| Lip vesicles | Negative | 0.1 | 0.4 | |
| Positive | 0.2 | 0.6 | 0.400 | |
| Lip pain | Negative | 0.2 | 0.5 | |
| Positive | 0.3 | 0.9 | 0.876 |
AM = arithmetic mean; SD = standard deviation; *Mann-Whitney test
In the analysis of their stress levels (low, moderate, high), we made comparison between a group of patients with cheilitis with at least one positive allergen in allergy tests (prick and patch) and another group of cheilitis patients without a proven allergen. By analyzing our 50 patients who underwent allergy tests, due to a small sample, two analyses were performed: (1) the categories of moderate and high stress were merged, and (2) the original three-stage categorization of stress was retained. Combining the categories of moderate and high stress, it was established that low-stress levels were less frequent in patients with confirmed allergies than in non-allergic patients, but as the sample of non-allergic patients was small, the difference was not statistically significant. Also, the largest differences in the prevalence of psychological stress were observed for moderate stress which was more common in patients with allergies, followed by low stress which was slightly more common in non-allergic patients, but the differences were not significant due to a small sample of non-allergic patients (Table 3).
Table 3. Relationship between stress and allergy (N=50).
| Allergy | Total | p | τb* | |||
|---|---|---|---|---|---|---|
| Stress | No (N=8) | Yes (N=42) | ||||
| Low | n (%) | 4 (50%) | 7 (16.7%) | 11(22%) | ||
| Moderate | n (%) | 3 (37.5%) | 32 (76.2%) | 35 (70%) | ||
| High | n (%) | 1 (12.5%) | 3 (7.1%) | 4 (8%) | 0.079 | 0.204 |
*Kendall’s effect size in χ2-test
As for the results of the analysis of literature reviews on cheilitis, it was established that the latest research on cheilitis and possible related allergies primarily included data on the results of allergy tests, percentage of subjects testing positive in patch tests and the most common allergens (18, 21-23) (Table 4). When we searched/browsed Pubmed MESH and entered the term ‘cheilitis’ and added ‘allergies’ as the cause of cheilitis, we found 143 related articles (dated March 17, 2020). Almost all of them dealt with contact allergic reactions, and a few of them dealt with the problem of angioedema. Therefore, patients predominantly underwent patch testing and the information on atopy was obtained from their histories. As most of the research found in the literature deals with allergic contact cheilitis, the most common allergens that cause it and their prevalence among other forms of cheilitis are listed.
Table 4. Significant recent studies involving subjects with inflammatory lesions on the lips (cheilitis) related to allergies.
| Research | Subjects (N) | Methods | Results |
|---|---|---|---|
|
Budimir et al. (2019) Oral Surg Oral Med Oral Pathol Oral Radiol (18) |
30 subjects with cheilitis (total 230 subjects, 180 with oral and perioral diseases and 50 healthy controls) | SPT (standard series of allergens) and PT (standard series of allergens and dental screening series) | Allergic reactions were most often observed in patients with cheilitis (60%). The most common forms of cheilitis: angular, simplex, exfoliative, contact, granulomatous. 43.3% were positive in SPT, the most common positive allergens: Inhalants: grass pollen Preservatives and additives: glutaraldehyde, citric acid Food: fruits In PT, 26.7% were positive, the most common allergens being cobalt chloride, nickel sulfate and mercury precipitate. |
|
Cheng et al. (2019) Dermatitis (21) |
91 subjects with cheilitis | Health data from 2 private clinics, PT (standard series of allergens); retrospective research |
Patients with cheilitis were most often/usually younger and atopic women. Allergic contact cheilitis was found in 17% of the subjects with cheilitis. It was established that the most common allergic reactions were reactions to the patients’ own products, nickel sulfate, balsam of Peru, fragrance mix, benzophenone and cobalt chloride. The patients with cheilitis were more likely to have positive allergic reactions to sunscreen ingredients (benzophenones) than patients without cheilitis. |
|
O’Gorman et al. (2016) Int J Dermatol (22) |
91 subjects with cheilitis | Health data from register, PT (standard series of allergens and allergens of patients’ own products); retrospective research |
77% of women; average age: 51 Definitive diagnoses: Contact allergic cheilitis: 45% Contact irritant cheilitis: 11% Atopic cheilitis: 3% Other: 22% (granulomatous cheilitis 10, exfoliative cheilitis 3, glandular cheilitis 1, pyostomatitis vegetans 1, erythema multiforme 1, lymphedema 1, BMS 1, lichen 1, reaction to esomeprazole 1); Unknown: 19%. The most common positive reactions in patients with contact cheilitis were those to fragrance mix, balsam of Peru, dodecyl gallate, nickel sulfate, golden sodium thiosulfate, octyl gallate. |
|
Kim et al. (2015) Ann Dermatol (23) |
12 subjects with cheilitis (total 44 subjects with oral diseases) | Health data from register, PT (dental screening series); retrospective research |
The prevalence of cheilitis was 27.3% (being the second most common). 75% of the subjects were positive to dental allergens. The most common positive reactions were to cobalt chloride hexahydrate, potassium dichromate, nickel sulfate, mercury, and one positive reaction to palladium chloride and gold sodium thiosulfate each. |
SPT = standard prick test; PT = patch test; BMS = burning mouth syndrome
Discussion
According to the literature, cheilitis is one of the most common oral manifestations of a contact allergic reaction, and the patch test performed with standard contact allergens but also with possible allergens of patients’ own products may be very useful for patients (1). In terms of the prevalence of allergies to contact allergens, Torgerson et al. found that 25.9% of subjects with cheilitis had at least one positive reaction in patch test (24), Budimir et al. observed it in 26.7% (18), Khamaysi et al. in 41.9% (25), and Kim et al. even in 75% (23) of subjects. In our study, 54% of the subjects with cheilitis had at least one positive reaction to allergens in the patch test to standard series and dental series, similar as in the study by Zoli et al. (54.2%) (26). In the research by Budimir et al. (testing with the standard series of allergens and additional allergens depending on medical history) and Kim et al. (dental series allergen testing), the most common allergens detected by the patch test were cobalt chloride, nickel sulfate, and mercury precipitate (18, 23), similar as in our study where the most common positive reaction was also to cobalt chloride and nickel sulfate, followed by thimerosal. In the study by Zoli et al. (the standard series of allergens), the results were similar to ours and the most common allergen was nickel sulfate, followed by thimerosal and cobalt chloride (26). Nickel sulfate was the most common allergen in the study by Lim et al. (the standard series of allergens and the patients’ own products), followed by the patients’ own products and ricinoleic acid (27). According to the research by Cheng et al. (testing to the standard series of allergens), nickel sulfate ranked second in terms of prevalence (immediately behind the patients’ own products); it was followed by balsam of Peru, fragrance mix, benzophenone, and cobalt chloride (21). This suggests that nickel sulfate and cobalt chloride are among the most common allergens in patients with cheilitis (cobalt sensitization is usually associated with nickel sensitization) (28). The use of cobalt chloride is widespread; it is used in chemical and pharmaceutical industries, as an additive to dyes, in vitamin preparations and as an additive to animal feed (28). Also, this material is often used in dentistry, just like nickel. Nickel is a metal found all around us, including drinking water, fertilizers, food, paints, dishes, cutlery, jewelry, etc. Clearly, avoiding it in our everyday life is almost impossible. The third most common allergen in patients with cheilitis in our study was thimerosal, which is used as a preservative in various cosmetic and ophthalmic preparations and vaccines (28).
According to the literature, aside from cheilitis, some other oral diseases are also associated with contact allergens, e.g., gingivitis, stomatitis, perioral dermatitis, burning mouth syndrome, lichenoid reaction, and orofacial granulomatosis (29). According to Budimir et al., 60% of patients with cheilitis had at least one positive allergic reaction, so cheilitis ranked first, leaving behind other examined diseases (burning mouth syndrome, angioedema, oral lichen planus, gingivostomatitis, and perioral dermatitis) (18, 29). According to Kim et al., the most frequent allergic reactions were those in patients with cheilitis (75%, as well as in a group of patients with oral lichen planus) (23). Generally, patients with cheilitis showed positive reactions to contact allergens (i.e., delayed hypersensitivity reactions) more often than patients with other abovementioned oral diseases (18). According to Khamaysi et al., the prevalence of allergic reactions in patients with cheilitis and perioral dermatitis ranked third (41.9% of subjects tested positive in patch test for allergens in a dental series), just behind orofacial granulomatosis and burning mouth syndrome (other analyzed groups included those with hand eczema in dental occupations, oral lichen planus, glossodynia, recurrent aphthous stomatitis, and other diseases) (25). Gender is also important. Allergic contact cheilitis is more common in women because they generally use more cosmetics, are more aware of changes in their appearance, and are more likely to seek medical help than men (22). Also, the prevalence of this form of cheilitis increases with age, which is associated with increased use of hygienic and cosmetic products (26). Clinical manifestations of allergic contact cheilitis include dryness, peeling and fissures with erythema, more often on perioral skin where circumoral edema is possible (1, 10), while the extent of the lesion depends on the cause. In food-induced cheilitis, inflammation very often spreads to the skin around the lips. If cheilitis is caused by an allergic reaction to a small object that comes in contact with lips (e.g., needles, pencils, hairpins), then the lesion is limited to that part of the lips (12). Allergic contact cheilitis may manifest with itching, burning and lip hardening, and even with consequent lip hyperpigmentation (12, 30). The fact that the prevalence of allergic reactions in patients with cheilitis was higher than in those with other oral diseases indicates the importance of allergic testing in these patients. The higher prevalence of allergies observed in patients with cheilitis than in those with other oral diseases can be explained by several factors. Due to keratinization of the lips, the allergen may persist deeper in the corneal layer and the keratin layer is better supplied with hapten-bound proteins. Also, saliva dilutes, washes away and digests a certain amount of antigen, and there are fewer Langerhans cells in the mucosa than in the skin. Due to all the above, mucous membrane is more difficult to sensitize than the skin or lips (4).
In addition to patch tests, according to Holmes et al., prick tests for examination of IgE-mediated reactions should be applied in practice to reveal/confirm eczematous or contact allergic cheilitis (31). This form of allergic reaction occurs as a result of repeated contacts with a particular allergen (food, preservatives, odors) over time and leads to contact urticaria (31). One article describes a case of a patient with confirmed contact urticaria to toothpaste mint, where the prick test was positive for mint leaves, while all patch tests were negative (31). The prick test for determination of food allergens is important for diagnosing the so-called food cheilitis, or allergic reactions to food manifested on the lips (13). Performing a prick test for inhaled allergens is also important for determining possible atopy and confirming or dismissing the suspicion of atopic cheilitis. Such patients with a history of cheilitis usually have atopic diathesis (involving asthma, allergic rhinitis and dermatitis) and also eosinophilia and elevated serum IgE (1). At the same time, we support the introduction of prick test into the diagnostic practice for cheilitis, in addition to patch test that has been predominantly used (22-24, 26, 27, 32). It is notable that in these previous studies, data on atopy were obtained exclusively from patient history. The percentage of atopics in the study by Freeman et al. was 19% (32), while in the study by Lim et al., atopy was found in one-third of the subjects (27); similar percentage is reported by Zoli et al. (34.9%) (26). According to our results, the percentage of atopics is even higher (84%), which can be explained by the fact that the study was conducted at the Department of Dermatovenerology, which includes the Allergy Unit.
Based on scarce information on immediate allergic hypersensitivity (prick test) in patients with cheilitis found in the literature, Budimir et al. established that reactions to inhalant allergens (indicating atopic constitution of the patient) were demonstrated in 30% of the subjects with cheilitis (most commonly to weed pollen, grass pollen, and dust), which is consistent with our results (18). Food allergens were proven in 13.3% of the subjects, the most common being those to fruits and vegetables. Preservatives and additive allergens were proven in 20% of the subjects, and the most common were glutaraldehyde, glutamate, and citric acid (33). In our study, the most commonly proven allergens were inhalants (48%), while food allergens were proven in 18% of the subjects, among which wheat flour and pineapple were most common. Preservatives and additives as allergens were found in only 6% of cases. Like the study by Budimir et al., our study also established that the most common allergens among preservatives were glutaraldehyde and citric acid, followed by sodium benzoate. However, after obtaining a positive test, the clinical relevance of this allergy in patients yet remains to be determined in further procedure. For this reason, our subjects with allergic reactions established in allergy tests were advised to avoid these allergens in order to determine the relevance (significance) of the allergen for the disease itself.
Frequent occurrence of lesions and symptoms on the lips and, sometimes, their frequent recurrence, significantly trigger psychological stress and affect the quality of life in patients with cheilitis. Association between allergies and psychological stress has already been mentioned in the literature (34, 35). As regards our results on psychological stress in patients with cheilitis, moderate stress was more common in our patients with allergies, whereas in non-allergic patients, low stress was slightly more frequent, although the differences were not significant. In our patients with confirmed allergies, low stress levels were recorded less frequently than in non-allergic patients, but the difference was not statistically significant, probably due to the small sample.
Finally, the limitations of this study included several factors, as follows: we did not follow our patients after their allergy tests, so we did not have data if allergies established in the tests were relevant for their condition. Also, we considered a patient to be atopic only if the allergy test was positive (although more criteria are needed).
Conclusion
The most common allergens identified in the patients with cheilitis are inhalant allergens, as proven by the prick test, which indicates frequent atopy (allergy constitution) in the people with cheilitis. Common contact allergens also include cobalt chloride, nickel sulfate and thimerosal confirmed by patch test. The fact that atopy was found in 84% of the patients with cheilitis indicates a higher incidence of cheilitis in the people prone to allergies; it also indicates association between cheilitis and allergies.
References
- 1.Greenberg SA, Schlosser BJ, Mirowski GW. Diseases of the lips. Clin Dermatol. 2017;35(5):e1–14. 10.1016/j.clindermatol.2017.11.003 [DOI] [PubMed] [Google Scholar]
- 2.Oakley A. Cheilitis. Cheilitis | DermNet [Internet]. 2010 [cited 2020 Apr 3]. [about 1 p.]. Available from: https://www.dermnetnz.org/topics/cheilitis/.
- 3.Hitz Lindenmüller I, Itin PH, Fistarol SK. Dermatology of the lips: inflammatory diseases. Quintessence Int. 2014;45(10):875–83. 10.3290/j.qi.a32638 [DOI] [PubMed] [Google Scholar]
- 4.Alajbeg I. Oralne bolesti prema topografskoj klasifikaciji. In: CekiÊ-Arambaπin A, editor. Oralna medicina. Zagreb: ©kolska knjiga; 2005. p. 300-28. (in Croatian) [Google Scholar]
- 5.StatPearls [Internet]. Treasure Island: StatPearls Publishing; 2020 [modified 2020 Feb 12; cited 2020 May 27]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470592/.
- 6.Japundžić I, Novak D, Kuna M, Novak-Bilić G, Lugović-Mihić L. Analysis of dental professionals’ and dental students’ care for their skin. Acta Stomatol Croat. 2018;52(1):46–52. 10.15644/asc52/1/7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akoglu G, Yavuz SO. Coexistence of cheilitis glandularis and lichen planus: remarkable response to anti-inflammatory treatments. Indian J Dermatol. 2017;62(5):549. 10.4103/ijd.IJD_581_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaga M, Nakamoto Y, Nakamura K, Ikeda K, Yoshii M, Kawana S. Stress sensitivity in patients with atopic dermatitis in relation to the translocator protein 18 kDa (TSPO). J Nippon Med Sch. 2014;81(3):148–56. 10.1272/jnms.81.148 [DOI] [PubMed] [Google Scholar]
- 9.Hanifin JM, Rajka G. Diagnositic features of atopic dermatitis. Acta Derm Venereol. 1980;92:44–7. 10.2340/00015555924447 [DOI] [Google Scholar]
- 10.Lugović-Mihić L, Pilipović K, Crnarić I, Šitum M, Duvančić T. Differential diagnosis of cheilitis: how to classify cheilitis? Acta Clin Croat. 2018;57(2):342–51. 10.20471/acc.2018.57.02.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.StatPearls [Internet]. Treasure Island: StatPearls Publishing; 2020 [modified 2020 May 9; cited 2020 Jul 21]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK536929/.
- 12.Scully C. Dermatoses of the oral cavity and lips. In: Griffiths C, Barker J, Bleiker T, Chalmers R, Creamer D, editors. Rook’s Textbook of Dermatology. Vol. 3. 9th edn. Oxford: Wiley Blackwell; 2016. p. 110.1-110.94. [Google Scholar]
- 13.Collet E, Jeudy G, Dalac S. Cheilitis, perioral dermatitis and contact allergy. Eur J Dermatol. 2013;23(3):303–7. 10.1684/ejd.2013.1932 [DOI] [PubMed] [Google Scholar]
- 14.Critchlow WA, Chang D. Cheilitis granulomatosa: a review. Head Neck Pathol. 2014;8(2):209–13. 10.1007/s12105-013-0488-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thongprasom K. Glycerin borax treatment of exfoliative cheilitis induced by sodium lauryl sulfate: a case report. Acta Stomatol Croat. 2016;50(2):158–61. 10.15644/asc50/2/9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girijala RL, Falkner L, Dalton SR, Martin BD. Exfoliative cheilitis as a manifestation of factitial cheilitis. Cureus. 2018;10(5):e2565. 10.7759/cureus.2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johansen JD, Aalto-Korte K, Agner T, Andersen KE, Bircher A, Bruze M, et al. European Society of Contact Dermatitis guideline for diagnostic patch testing – recommendations on best practice. Contact Dermatitis. 2015;73(4):195–221. 10.1111/cod.12432 [DOI] [PubMed] [Google Scholar]
- 18.Budimir J, Mravak-Stipetić M, Bulat V, Ferček I, Japundžić I, Lugović-Mihić L. Allergic reactions in oral and perioral diseases – what do allergy skin test results show? Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;127(1):40–8. 10.1016/j.oooo.2018.08.001 [DOI] [PubMed] [Google Scholar]
- 19.Japundžić I, Vodanović M, Lugović-Mihić L. An analysis of skin prick tests to latex and patch tests to rubber additives and other causative factors among dental professionals and students with contact dermatoses. Int Arch Allergy Immunol. 2018;177(3):238–44. 10.1159/000490181 [DOI] [PubMed] [Google Scholar]
- 20.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–96. 10.2307/2136404 [DOI] [PubMed] [Google Scholar]
- 21.Cheng HS, Konya J, Lobel E, Fernandez-Penas P. Patch testing for cheilitis: a 10-year series. Dermatitis. 2019;30(6):347–51. 10.1097/DER.0000000000000524 [DOI] [PubMed] [Google Scholar]
- 22.O’Gorman SM, Torgerson RR. Contact allergy in cheilitis. Int J Dermatol. 2016;55(7):e386–91. 10.1111/ijd.13044 [DOI] [PubMed] [Google Scholar]
- 23.Kim TW, Kim WI, Mun JH, Song M, Kim HS, Kim BS, et al. Patch testing with dental screening series in oral disease. Ann Dermatol. 2015;27(4):389–93. 10.5021/ad.2015.27.4.389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torgerson RR, Davis MDP, Bruce AJ, Farmer SA, Rogers RS, 3rd. Contact allergy in oral disease. J Am Acad Dermatol. 2007;57(2):315–21. 10.1016/j.jaad.2007.04.017 [DOI] [PubMed] [Google Scholar]
- 25.Khamaysi Z, Bergman R, Weltfriend S. Positive patch test reactions to allergens of the dental series and the relation to the clinical presentations. Contact Dermatitis. 2006;55(4):216–8. 10.1111/j.1600-0536.2006.00905.x [DOI] [PubMed] [Google Scholar]
- 26.Zoli V, Silvani S, Vincenzi C, Tosti A. Allergic contact cheilitis. Contact Dermatitis. 2006;54(5):296–7. 10.1111/j.0105-1873.2006.0698b.x [DOI] [PubMed] [Google Scholar]
- 27.Lim SW, Goh CL. Epidemiology of eczematous cheilitis at a tertiary dermatological referral centre in Singapore. Contact Dermatitis. 2000;43(6):322–6. 10.1034/j.1600-0536.2000.043006322.x [DOI] [PubMed] [Google Scholar]
- 28.Tomljanović-Veselski M, Jovanović I. Najčešći kontaktni alergeni u bolesnika s kontaktnim dermatitisima u području Slavonskog Broda. Med Jadert. 2006;36(1-2):45–52. [cited 2020 August 7] Available from https://hrcak.srce.hr/10728 [Internet] [Google Scholar]
- 29.Bakula A, Lugović-Mihić L, Šitum M, Turčin J, Sinković A. Contact allergy in the mouth: diversity of clinical presentations and diagnosis of common allergens relevant to dental practice. Acta Clin Croat. 2011;50(4):553–61. [PubMed] [Google Scholar]
- 30.Lugović-Mihić L, Blagec T, Japundžić I, Skroza N, Delaš Adžajić M, Mravak-Stipetić M. Diagnostic management of cheilitis: an approach based on a recent proposal for cheilitis classification. Acta Dermatovenerol Alp Pannonica Adriat. 2020;29(2):67–72. 10.15570/actaapa.2020.16 [DOI] [PubMed] [Google Scholar]
- 31.Holmes G, Freeman S. Cheilitis caused by contact urticaria to mint flavoured toothpaste. Australas J Dermatol. 2001;42(1):43–5. 10.1046/j.1440-0960.2001.00472.x [DOI] [PubMed] [Google Scholar]
- 32.Freeman S, Stephens R. Cheilitis: analysis of 75 cases referred to a contact dermatitis clinic. Am J Contact Dermat. 1999;10(4):198–200. 10.1053/AJCD01000198 [DOI] [PubMed] [Google Scholar]
- 33.Budimir J. Značenje alergoloških kožnih testova u dijagnostici bolesti oralne i perioralne regije uz nespecifične oralne smetnje [dissertation]. Zagreb: School of Dental Medicine, University of Zagreb; 2016. 74 p. (in Croatian) [Google Scholar]
- 34.Meštrović-Štefekov J, Novak-Bilić G, Kuna M, Pap N, Lugović-Mihić L. Psychological stress in patients with atopic dermatitis. Acta Dermatovenerol Croat. 2018;26(4):297–303. [PubMed] [Google Scholar]
- 35.Marshall GD, Jr. Psychological stress, immune dysfunction, and allergy: opportunities for improved patient health. Ann Allergy Asthma Immunol. 2020;125(4):365–6. 10.1016/j.anai.2020.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]