Abstract
Megacystis-microcolon-intestinal hypoperistalsis syndrome is a rare congenital disease with a poor prognosis and life expectancy. We present the prenatal diagnosis of four consecutive cases in the same woman. After medical genetics consultation, the couple was advised to resort to medically assisted reproduction techniques with oocyte donation. This case report demonstrates the recurrence of a rare disease in four consecutive pregnancies and how prenatal diagnosis assumed a preponderant role in parental counseling.
Keywords: Berdon syndrome, megacystis, megacystis-microcolon-intestinal hypoperistalsis syndrome
INTRODUCTION
Megacystis-microcolon-intestinal hypoperistalsis syndrome (MMIHS) is a rare congenital disorder of smooth muscle.[1] It predominantly affects females (4:1 ratio)[2] and is characterized by decreased muscle tone in the urinary tract and intestine, resulting in abdominal distension. It is caused by a dilated nonobstructive hypotonic urinary bladder (megacystis) with upper urinary tract dilation and intestinal hypoperistalsis, causing a pseudo-obstruction with shortened and malrotated microcolon.[3]
MMIHS is usually diagnosed prenatally or shortly after delivery. Its prenatal suspicion is based on the presence of megacystis on fetal ultrasound (US). Prenatal diagnosis is challenging due to its rarity and inconsistency of characteristic sonographic findings.[4]
MMIHS is associated with significant postnatal morbimortality, representing a significant cause of functional intestinal obstruction. Clinical presentation resembles other neonatal intestinal obstructions: bile-stained vomiting and failure to pass meconium. The course of the disease frequently implicates surgery, total parenteral nutrition, and mortality, mainly caused by sepsis, malnutrition, or multiple organ failure.[5]
CASE REPORT
A 29-year-old G1P0 woman with an unremarkable medical history and no history of consanguinity. First-trimester US at 13 weeks revealed megacystis with 9 mm × 9 mm, an enlarged stomach [Figure 1a], and an omphalocele with 9.8 mm × 6 mm [Figure 1b].
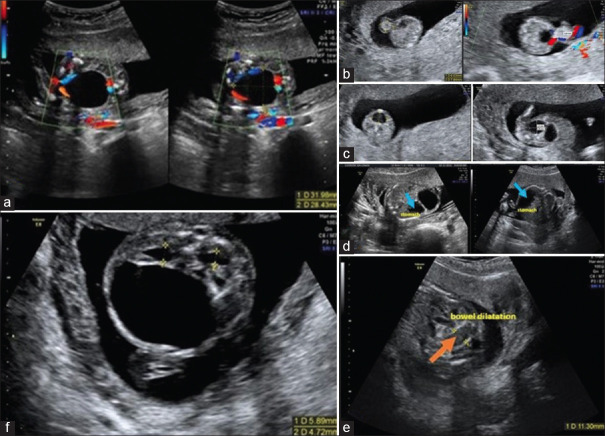
Figure 1.
(a) Enlarged stomach, (b) omphalocele with 9.8 mm × 6 mm, (c) megacystis with 38 mm × 31 mm, (d) gastric distension with 19 mm × 11 mm, (e) bowel dilation, (f) hyperechogenic kidneys and hydronephrosis
Chorionic villous sampling (CVS) was performed at 13+4 weeks, revealing a normal female comparative Genomic Hybridization (cGH) array. At 19+4 weeks, US demonstrated, megacystis with 38 mm × 31 mm [Figure 1c], gastric distension with 19 mm × 11 mm [Figure 1d], bowel dilation [Figure 1e], hyperechogenic kidneys and hydronephrosis [Figure 1f] and adequate fetal biometries for gestational age.
Pregnancy termination was required, accepted, and performed at 20 weeks gestation, according to Portuguese law.
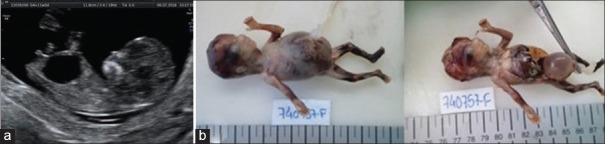
The autopsy confirmed megacystis [Figure 2a], hydroureter and hydronephrosis [Figure 2b], microcolon [Figure 2c], duodenomegaly, and several dilations of the small bowel associated with bowel ganglionic dysplasia/hyperplasia, suggesting MMIHS.
Figure 2.

Second pregnancy – (a) megacystis, (b) hydroureter and hydronephrosis, (c) microcolon
Sequestration of the ACTG2 gene showed no mutations.
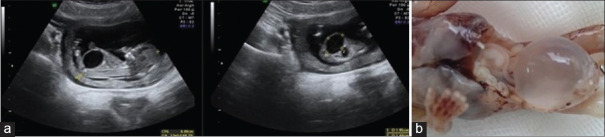
In the second pregnancy, the first-trimester US at 11+6 weeks showed a megacystis [Figure 3a]. CVS was revealing normal male array cGH. Given the likelihood of recurrence of the MMIHS, the parents requested for pregnancy termination at 13 weeks. The autopsy demonstrated a male fetus with megacystis [Figure 3b], hydroureter, microcolon, and bowel ganglionic dysplasia, supporting the hypothesis of MMIHS. Whole exome sequencing in a trio was normal.
Figure 3.
Third pregnancy – (a) megacystis, (b) male with megacystis
The 13-week scan of the third pregnancy also detected fetal megacystis [Figure 4a], hyperechogenic kidneys, hydronephrosis, and gastric distension, identical to previous pregnancies. The pregnant declined CVS and requested pregnancy termination. The autopsy revealed a male fetus, with 13 weeks, rectus muscle hypoplasia, megacystis and hydronephrosis, and a distended stomach without microcolon [Figure 4b].
Figure 4.
Fourth pregnancy – (a) megacystis, (b) megacystis and hydronephrosis and a distended stomach without microcolon
On the fourth pregnancy, first-trimester US had similar findings. The autopsy identified megacystis, hydronephrosis, and urethral valves, suggesting low urinary tract obstruction.
After a new genetic appointment, the couple was advised to do in vitro fertilization (IVF) with oocyte donation, given that an epigenetic etiology might be implicated in this high recurrence rate. IVF treatment with oocyte donation was performed, resulting in a dichorionic twin pregnancy and two healthy newborns at term.
DISCUSSION
MMIHS is a severe congenital visceral myopathy whose etiology remains unclear. Some genes related to the syndrome, such as ACTG2 (the most frequent), MYH11, LMOD1, and MYLK, encode proteins involved in smooth muscle contraction, supporting a myopathic basis for the disease. Most of the published cases of MMIHS are sporadic and caused by de novo heterozygous variants in ACTG2.[6,7] This syndrome has also been reported in offspring of a consanguineous marriage or various siblings with healthy parents.[8] The hypothesis of gonadal mosaicism has been reported in cases of recurrence among siblings of asymptomatic parents with no identified variant in the sequencing.[7,9]
Megacystis (longitudinal bladder dimension of ≥7 mm in the first trimester) is the most common sign of prenatal suspicion of MMIHS.[10] In this case, megacystis was present in four gestations. This is in line with the literature, which reported megacystis as an initial finding of MMIHS in 88% of individuals.[4] A dilated bladder may be due to obstructive or nonobstructive anomalies. Among obstructive causes of megacystis, it should be considered posterior urethral valves (PUVs), urethral atresia/stenosis, and cloacal anomalies. Regarding the first pregnancy, cloacal anomalies stood as a differential diagnosis since they are more frequent in females. PUV was a less likely cause because it occurs typically in male fetuses. Nonobstructive anomalies are related to impaired vesical tone and are frequently associated with neurological, genetical, and chromosomal disorders and prune belly syndrome. It is difficult to consistently distinguish between obstructive causes of fetal megacystis and MMIHS since the “keyhole” sign corresponds to a dilated proximal urethra which is more typical of a mechanical lower urinary tract obstruction.[4]
Because of the severity and broad spectrum of clinical presentations related to ACTG2 mutation, it is not possible to define a genotype–phenotype correlation.[7] In this case, although similar phenotypes between gestations, no mutations were found. In the first pregnancy, the study of the array and sequencing of the ACTG2 gene did not reveal alterations. The suspicion of MMIHS in the second pregnancy was promptly considered because of megacystis and the previously affected sister. However, array and trio exome sequencing did not show any changes. Given the circumstances, geneticists considered the empirical risk of recurrence as significantly increased. In the absence of an etiological diagnosis, epigenetic etiology was assumed as a possibility. To minimize the risk of maternal gonadal mosaicism, IVF with oocyte donation was proposed.
According to the scarce literature supported by individual case reports or small case series, the prenatal US diagnosis and etiology of MMIHS remain challenging. This case demonstrates the MMIHS phenotypic heterogeneity since it recurred four times in the same couple (nonconsanguineous and without identified ACTG2 mutations). Attending the possibility of gonadal mosaicism and the difficulty in providing parental counseling, this case also highlights the importance of the anatomopathological examination of the fetus, in confirming the prenatal suspicion, for the counseling of subsequent pregnancies.
Given its rarity, the great variability in US findings and clinical status, histological changes and prognosis with mortality, further studies are needed for a better genetic classification of MMIHS.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank the Center of Prenatal Diagnosis, Centro Hospitalar Vila Nova de Gaia/Espinho, Vila Nova de Gaia, Portugal.
REFERENCES
- 1.Ignasiak Budzyńska K, Danko M, Książyk J. Megacystis microcolon intestinal hypoperistalsis syndrome (MMIHS): Series of 4 cases caused by mutation of ACTG2 (actin gamma 2, smooth muscle) gene. Case Rep Gastrointest Med 2021. 2021:6612983. doi: 10.1155/2021/6612983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.López-Muñoz E, Hernández-Zarco A, Polanco-Ortiz A, Villa-Morales J, Mateos-Sánchez L. Megacystis-microcolon-intestinal hypoperistalsis syndrome (MMIHS): Report of a case with prolonged survival and literature review. J Pediatr Urol. 2013;9:e12–8. doi: 10.1016/j.jpurol.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 3.White SM, Chamberlain P, Hitchcock R, Sullivan PB, Boyd PA. Megacystis-microcolon-intestinal hypoperistalsis syndrome: The difficulties with antenatal diagnosis. Case report and review of the literature. Prenat Diagn. 2000;20:697–700. doi: 10.1002/1097-0223(200009)20:9<697::aid-pd891>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 4.Tuzovic L, Anyane-Yeboa K, Mills A, Glassberg K, Miller R. Megacystis-microcolon-intestinal hypoperistalsis syndrome: Case report and review of prenatal ultrasonographic findings. Fetal Diagn Ther. 2014;36:74–80. doi: 10.1159/000357703. [DOI] [PubMed] [Google Scholar]
- 5.Prathapan KM, King DE, Raghu VK, Ackerman K, Presel T, Yaworski JA, et al. Megacystis microcolon intestinal hypoperistalsis syndrome: A case series with long-term follow-up and prolonged survival. J Pediatr Gastroenterol Nutr. 2021;72:e81–5. doi: 10.1097/MPG.0000000000003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Billon C, Molin A, Poirsier C, Clemenson A, Dauge C, Grelet M, et al. Fetal megacystis-microcolon: Genetic mutational spectrum and identification of PDCL3 as a novel candidate gene. Clin Genet. 2020;98:261–73. doi: 10.1111/cge.13801. [DOI] [PubMed] [Google Scholar]
- 7.Moreno CA, Metze K, Lomazi EA, Bertola DR, Barbosa RH, Cosentino V, et al. Visceral myopathy: Clinical and molecular survey of a cohort of seven new patients and state of the art of overlapping phenotypes. Am J Med Genet A. 2016;170:2965–74. doi: 10.1002/ajmg.a.37857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alnoaiji MS, Ghmaird AS, Alrashidi TN, Alqoaer KI, Abdelmalek RW, Alshahrani EH. Megacystis microcolon intestinal hypoperistalsis syndrome in two sisters with a rare disease gene. Med Sci. 2021;25:954–8. [Google Scholar]
- 9.Tuzovic L, Tang S, Miller RS, Rohena L, Shahmirzadi L, Gonzalez K, et al. New insights into the genetics of fetal megacystis: ACTG2 mutations, encoding ?-2 smooth muscle actin in megacystis microcolon intestinal hypoperistalsis syndrome (Berdon syndrome) Fetal Diagn Ther. 2015;38:296–306. doi: 10.1159/000381638. [DOI] [PubMed] [Google Scholar]
- 10.Buinoiu N, Panaitescu A, Demetrian M, Ionescu S, Peltecu G, Veduta A. Ultrasound prenatal diagnosis of typical megacystis, microcolon, intestinal hypoperistalsis syndrome. Clin Case Rep. 2018;6:855–8. doi: 10.1002/ccr3.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]