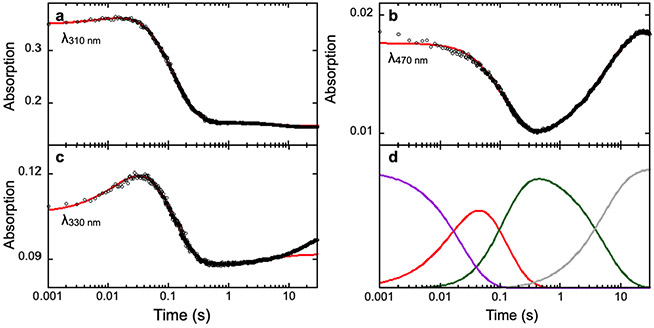
Fig. 2.
SF-Abs derived kinetic traces of the time-dependent absorption changes at various wavelengths in the AsqJ-catalyzed epoxidation by using 2-H as the substrate. Experimental data are shown in black dots, the kinetic simulations are shown in red curves. The resulting kinetics of individual species from the simulations are shown in panel d with the following color codes: purple, the AsqJ•Fe(II)•2OG•2-H quaternary complex; red, the oxyferryl intermediate; green, the AsqJ•Fe(II)•sucinnat•product complex; gray, the AsqJ•Fe(II)•2OG ternary complex. The figure is adapted with permission from Li, J., Liao, H.-J., Tang, Y., Huang, J.-L., Cha, L., Lin, T.-S., … Guo, Y. (2020). Epoxidation catalyzed by the nonheme iron(II)- and 2-oxoglutarate-dependent oxygenase, AsqJ: Mechanistic elucidation of oxygen atom transfer by a ferryl intermediate. Journal of the American Chemical Society, 142(13), 6268–6284. https://doi.org/10.1021/jacs.0c00484 Copyright 2020 American Chemical Society.