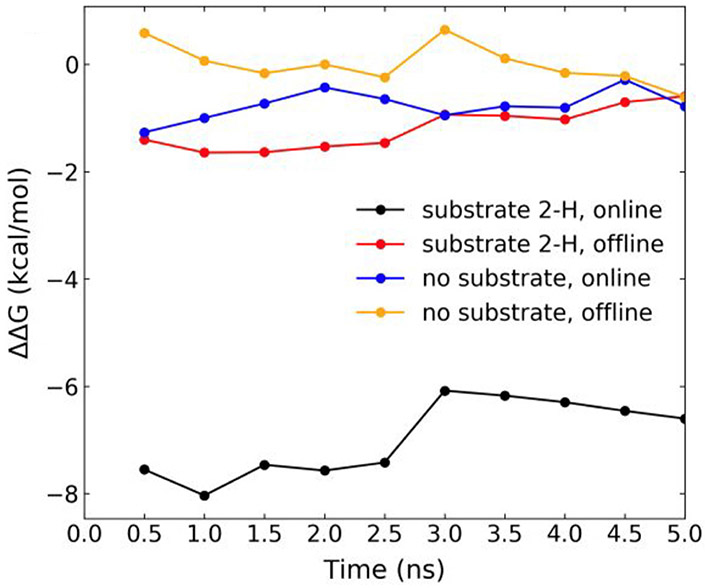
Fig. 6.
Free energy differences (ΔΔG) for H2O to O2 replacement at the Fe(II) center of AsqJ in two different 2OG binding configurations derived from MD simulations. Cumulative ΔΔG for four system configurations (see Scheme 3 for the illustration of the four system configurations) averaged over 0.5 ns MD simulation intervals by using the 2OG offline configuration without substrate as the reference state. Simulation for each lambda runs for 5 ns. The BAR method calculates free energy difference along the 5 ns trajectories. We plot the running average over 0.5 ns in the figure. For black trace, ΔΔG = ΔG4–ΔG1, for the red trace, ΔΔG = ΔG3–ΔG1, for the blue trace, ΔΔG = ΔG2–ΔG1, and for the orange trace, ΔΔG = ΔG1–ΔG1. See the definition of ΔGi (i = 1–4) in Scheme 4. The figure is adapted from Li, J., Liao, H.-J., Tang, Y., Huang, J.-L., Cha, L., Lin, T.-S., … Guo, Y. (2020). Epoxidation catalyzed by the nonheme iron(II)- and 2-oxo-glutarate-dependent oxygenase, AsqJ: Mechanistic elucidation of oxygen atom transfer by a ferryl intermediate. Journal of the American Chemical Society, 142(13), 6268–6284. https://doi.org/10.1021/jacs.0c00484 Copyright 2020 American Chemical Society.