Summary
Background
Only limited information exists regarding the epidemiology of Kawasaki disease (KD) in low-income and middle-income countries. The present study provides the incidence of KD during 2015–2019 in Chandigarh, north India. Our centre follows the largest KD cohort in India.
Methods
Children with KD at Chandigarh diagnosed during January 2015–December 2019 were enrolled in the study. Annual incidence rates were determined using decadal growth rates of the National Census 2011. We computed the incidence of KD in children aged <5, and <15 years. We also undertook linear trend analysis using our incidence data from 1994 to 2019.
Findings
During 2015–2019, 83 patients (66 males, 17 females) were diagnosed with KD in Chandigarh. Incidence rates during these 5 years were 5.64, 9.25, 9.11, 9.87, and 9.72/100,000 in children aged <5 years, and 2.65, 4.44, 3.86, 5.07, 4.74/100,000 in children aged <15 years. The median age at diagnosis was 48 months (range: 12 days to 15 years). Compared to previous data (2009–2014), there was a 53.1% increase in annual incidence of KD in children aged <5 years, and a 53.7% increase in children aged <15 years. Coronary artery abnormalities during acute phase were noted in 16.9%, and in 7.2% of patients at 6 weeks of follow-up. The trend analysis indicated a monthly rise of 0.002 cases per 100,000 children aged <5 years, and 0.0165 cases per 100,000 children aged <15 years.
Interpretation
The incidence of KD has continued to show an upward trend in Chandigarh over the period 2015–2019. This may indicate a true rise in the occurrence of KD or may reflect better disease ascertainment as a result of greater awareness about KD amongst healthcare professionals.
Funding
None.
Keywords: Kawasaki disease, Awareness, Incidence, Epidemiology, Chandigarh, North India, Coronary artery abnormalities
Research in context.
Evidence before this study
Epidemiological data from Japan and high-income countries in North America and Europe show that Kawasaki disease (KD) has surpassed rheumatic fever to become the most common cause of paediatric-acquired heart disease. Only limited information is available regarding the incidence of KD in low-income and middle-income countries (LMICs). Anecdotal reports suggest a growing recognition of KD in India over the last two decades.
Added value of this study
This study shows that KD incidence in Chandigarh (a city and Union Territory in north India) has continued to show an upward trend over the period 1994–2019. The trend analysis indicated a monthly rise of 0.002 cases per 100,000 in children younger than 5 years, and 0.0165 cases per 100,000 in children younger than 15 years in Chandigarh. This may indicate a true rise in the incidence of KD or may reflect better disease ascertainment as a result of greater awareness about KD amongst healthcare professionals.
Implications of all the available evidence
KD, and not acute rheumatic fever, is now the most common cause of paediatric acquired heart disease in Chandigarh. There is a need to increase awareness about KD in India and other LMICs. Delays in diagnosis and treatment are associated with significant cardiac morbidity and mortality. A nationwide registry for KD is the need of the hour. The WHO acknowledges cardiovascular disease in their priority of actions. KD can now be considered a ‘priority disease’ by the WHO as the long-term morbidity associated with it can have significant implications for health planners.
Introduction
Kawasaki disease (KD) is a paediatric vasculitic disorder that primarily affects coronary arteries. KD usually occurs among children aged below 5 years but has also been reported in adolescents and adults.1,2 Epidemiological data from Japan, and high-income countries in North America, and Europe show that KD has displaced rheumatic fever (RF) to become the leading cause of paediatric-acquired heart disease.1,3,4 The reported incidence of KD in Japan before the COVID-19 pandemic was 371/100,000 children aged <5 years.5,6 In Japan, approximately 1 in 100 children develop KD by the first decade of life.6 South Korea and Taiwan have incidence rates of 194.7 and 69.5, respectively, while in the United States and Europe, the incidence ranges from 5 to 30/100,000 children aged <5 years.3,7, 8, 9 It is important to note that some Asian countries like Japan, Taiwan, and South Korea, have been reporting an increasing incidence of KD since the 1990s.8,10
Interestingly, very little is known about KD incidence in low-income and middle-income countries (LMICs) in Asia, Latin America, and Africa.11,12 Anecdotal reports suggest that KD is now being increasingly recognised in India over the last two decades.3,13, 14, 15 However, at present, there is no national-level registry for KD in India.
The first epidemiological study from our centre located in Chandigarh (a city and federally administered Union Territory [UT] in north India) documented the increasing incidence of KD from 1994 (0.51/100,000) to 2008 (4.54/100,000) in children aged <15 years.13 The second epidemiological study covered the period from 2009 to 2014, and reported the KD incidence rate of 5.35/100,000 children under the age of 5 years.16 In this study (which is continuation of our previous studies13,16), we have compared the incidence of KD during the period 2015–2019 with our previously published incidence figures. We have also analysed the linear trends in our incidence data during 1994–2019.
Methods
Study settings
This study was conducted in the Pediatric Allergy Immunology Unit, Advanced Pediatrics Centre, Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh, India. Our institute serves as a tertiary care teaching hospital in north India. Our unit is also a WHO Collaborating Centre for Education, Research, and Training in Paediatric Immunology. Chandigarh is bordered by the State of Punjab to the north and the west and the State of Haryana (District Panchkula) to the east.13,16,17
Data collection
Our centre follows the largest KD cohort in India. We have maintained a registry of all children diagnosed with KD since 1994. Records were reviewed for all children diagnosed with KD and residing in Chandigarh from Jan 1, 2015 to Dec 31, 2019. Data regarding admissions, age of presentation, and sex distribution were recorded for all cases of KD in Chandigarh who sought medical care at our hospital from 2015 to 2019. Diagnosis of KD was made based on guidelines of the American Heart Association.1,2 2D-transthoracic echocardiography (TTE) was carried out in all children during acute and follow-up stages. Before 2019, TTE was carried out on the Esaote (MyLab30Gold) ultrasonography machine. Since then, we have been using the Philips EPIQ 7G ultrasonography machine (model number US 51881625). TTE was usually carried out by postdoctoral fellows of our institute. Z-scores for coronary arteries were calculated using body surface area-based measurements given by Dallaire and colleagues1,18 Patients with significant coronary artery abnormalities (CAAs) on TTE also underwent CT coronary angiography (CTCA) on a 128-slice Dual Source platform with radiation optimization.19,20 All patients were treated as per standard treatment guidelines. Intravenous immunoglobulin (IVIg) resistance was defined as recrudescent or persistent fever at least 36 h following completion of the first dose of IVIg. Adjunctive therapy was administered to patients who had IVIg resistance, and also to patients who had CAAs at presentation.1,2
All procedures performed in studies involving human participants were following the ethical standards of the institutional and/or national research committee and with the Declaration of Helsinki (1964) and its later amendments or comparable ethical standards. The study was approved by the Departmental Review Board, Department of Pediatrics, Advanced Pediatrics Centre, PGIMER, Chandigarh (Approval No. DRB-14-24, 24.02.2024).
Estimation of incidence rate
To compute the incidence of KD, we followed the similar methods mentioned in our previous studies.13,16 To compute the incidence of KD, we have made the assumption that all children diagnosed to have KD in Chandigarh would have either reported to our centre directly, or would have approached for a second opinion after consultation elsewhere during the acute stage. However, it is still possible that some children with KD in Chandigarh may not have approached us for treatment or follow-up during the period of study. Therefore, the incidence figures emerging from our study might reflect the lowest possible incidence of KD at Chandigarh.
Estimates of the population of Chandigarh are based on the National Census conducted by the Registrar General and Census Commissioner of India every 10 years.21 However, the National Census could not be conducted in 2021 because of the COVID-19 pandemic (previous census was conducted in 2011). Therefore, we computed the population estimates for the subsequent years i.e., from 2012 to 2019 using the decadal growth rate of the National Census.
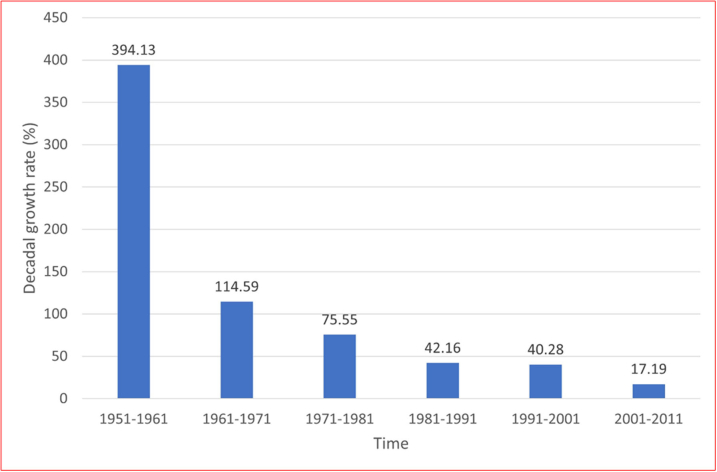
The population of Chandigarh was 1,054,686 in 2011, compared to 900,635 in 2001 - the population growth rate being 17.19%. During 2001–2011, there was a decline in the percentage growth rate of the population of Chandigarh as compared to previous decades (Supplementary Fig. S1). This demographic change is probably a reflection of increased literacy and improvement in the economic status of the population at large. The proportion of children aged 0–6 years has declined from 12.83% in 2001 to 11.18% in 2011. The number of children aged <6 years in Chandigarh in 2011 was 1,19,434. We further computed the estimates of children aged <5 years based on a pro-rata basis (i.e., by subtracting 1/6th). Therefore, population estimates for the subsequent years (i.e., from 2012 to 2019) were determined using the decadal growth rate of 17.19% derived from National Census data.
Seasonal variation
We calculated the seasonal distribution of KD cases at our centre during 2015–2019. Considering recent trends, Chandigarh has the following seasons: (i) Quarter 1- winter (January–February)/spring (March), (ii) Quarter 2- summer (April–June), (iii) Quarter 3- monsoon (July–August)/autumn (September), and (iv) Quarter 4- autumn (October)/winter (November–December). The trend estimation was performed using the moving average method. The seasonal variations were computed using the ratio to moving average method (Supplementary Table S1).
Comparison of incidence of KD and rheumatic fever
We also compared the estimated incidence of RF with that of KD in Chandigarh. Estimates of RF have been derived from a report of the ‘Jai Vigyan Mission Mode Project on Rheumatic Fever and Rheumatic Heart Disease’ funded by the Indian Council of Medical Research (ICMR), Ministry of Health and Family Welfare, Government of India.22 The data on the incidence of RF was available yearly for the period 2001–2008. Therefore, the incidence of RF and KD was compared using yearly data obtained during 2001–2008.
Data analysis
We have analysed the records of all children with KD from Chandigarh during 2015–2019. The incidence of KD was calculated based on the number of cases in both age groups (i.e., <5 years, and <15 years) residing at Chandigarh, as the numerator and the population of Chandigarh in the corresponding age group as the denominator. Separate incidence rates were computed for child population aged <5 years, and <15 years.
We have undertaken trend analysis for incidence of KD cases for the age <5 years and <15 years. The trends analysis of KD cases for both categories was performed to understand the fluctuation of KD cases over the considered timeframe. A p-value < 0.05 was considered as statistically significant.
Role of the funding source
There was no funding source for this study.
Results
Demographic characteristics
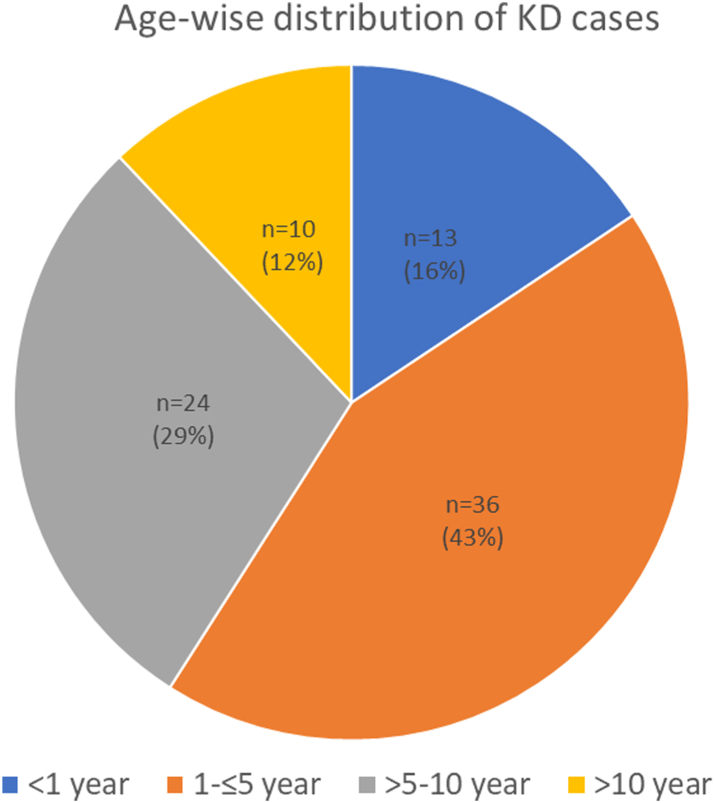
Between January 2015 and December 2019, of the 539 children with KD enrolled at our centre, 83 (15.4%; 66 males, 17 females) were from Chandigarh. The distribution of children with KD by age group is depicted in Fig. 1. The median age at diagnosis was 48 months (range: 12 days to 15 years). The median age at diagnosis for males and females were 4 and 5 years, respectively. A peak incidence was seen in the fourth year of life. The median interval between the onset of fever and diagnosis of KD was 9.4 days (range: 5–28).
Fig. 1.
Age-wise distribution of children with Kawasaki disease (KD) in Chandigarh, India during 2015–2019.
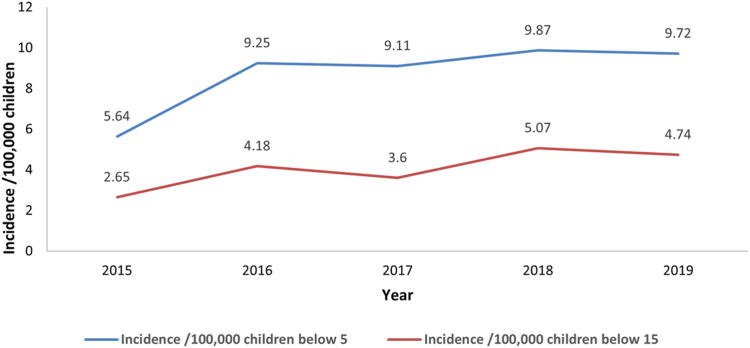
The mean incidence of KD during 2015–2019 was estimated at 8.89/100,000 children aged <5 years, and 4.25/100,000 children aged <15 years. During the years 2015–2019 (five years), the annual incidence rates of KD were 5.64, 9.25, 9.11, 9.87, and 9.72 per 100,000 children aged <5 years, respectively. The corresponding figures among children aged <15 years were 2.65, 4.18, 3.6, 5.07, and 4.74 per 100,000 children, respectively. The trend line depicted a notable increase in KD cases from 2015 to 2019 (Fig. 2). The occurrence of KD exhibited variations from year to year, and the highest incidence was observed in 2018, with a rate of 9.87/100,000 children aged <5 years and 5.07/100,000 children aged <15 years (Fig. 2).
Fig. 2.
Incidence of Kawasaki disease among children aged <5 and <15 years in Chandigarh, India (2015–2019). Below 5: children aged <5 years, below 15: children aged <15 years.
Seasonal variations and KD cases
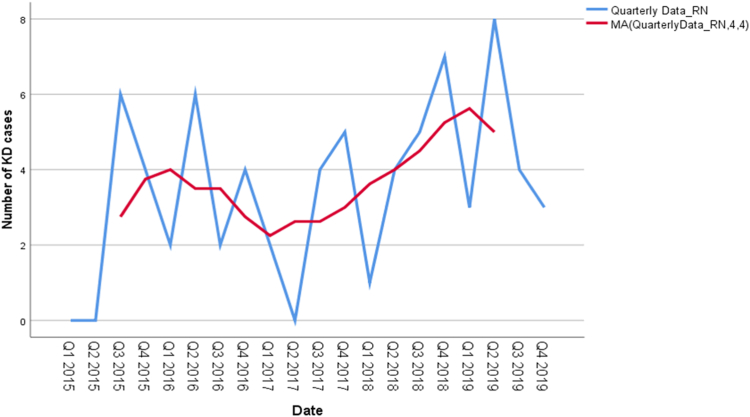
Some clustering of cases was noted in April (10 patients), September (10 patients) and August (9 patients). A nadir (two patients) was seen in February, whereas October, November, and December had 8 patients each. The seasonal variations are computed with the help of ratio-to-moving average method. The estimates of the seasonal indices indicate that the KD cases are increasing in Quarter 2 (April–June) and Quarter 4 (October–December) respectively. Specifically, the number of KD cases is 16% and 29% higher than the seasonal averages in Quarter 2 and Quarter 4, respectively (Fig. 3 and Supplementary Table S1).
Fig. 3.
Seasonal distribution of Kawasaki disease (KD) cases in Chandigarh during 2015–2019. The X-axis represents the quarters from January 2015 to December 2019. Red line depicts the number of KD cases estimated with the help of moving average methods (MA).
Clinical features
Complete KD was diagnosed among 48 children (57.8%), while incomplete KD was observed in 35 children (42.2%). Three children had a recurrence of KD. Chromonychia was observed in six patients (7.2%) and arthritis in two patients (2.4%). KD shock syndrome was observed in 6 (7.2%) cases. In 10 (12.1%) patients, KD was associated with concurrent infection. There was no mortality in this cohort.
Treatment details
The first-line therapy for KD were IVIg (2 g/kg) and oral aspirin (initially 30–50 mg/kg/day; and later 3–5 mg/kg/day). Low-dose aspirin (3–5 mg/kg/day) was continued in all patients for 4–6 weeks after the acute episode. Aspirin was stopped in children who had normal echocardiography findings at 6 weeks, whereas aspirin was continued in children with residual CAAs. Adjunctive therapy was required in seven patients: infliximab in four patients, oral prednisolone in two patients, and one patient received a combination of infliximab, cyclosporine A, and oral prednisolone. There were no treatment-related complications in our cohort. Nine patients (11.4%) were not given treatment as they had presented during convalescence and had already become afebrile, inflammatory biomarkers had settled and TTE showed no evidence of coronary involvement.
Cardiovascular complications
CAAs were detected in 14 patients (16.9%) during the acute phase of the disease: in the left anterior descending artery in six patients, in right coronary artery in four patients, and in left main coronary artery in two patients. Two patients had increased coronary artery brightness along with loss of tapering. Two patients had symptomatic myocarditis along with CAAs. At 6 weeks of follow-up, six patients (7.2%) had residual CAAs. Two patients had >1 Z score change in diameter at 6 weeks of follow-up. CTCA was performed in seven patients and showed CAAs in two patients.
Comparison of incidence of KD at Chandigarh to previously published data, and trends
We have compared the KD incidence at Chandigarh during 2015–2019 with previously published epidemiological data from our centre. Our findings indicate a notable increase in the incidence of KD in the current study compared to our previously published incidence data.13,16
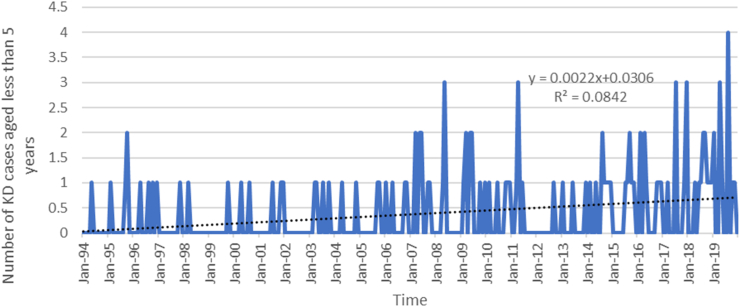
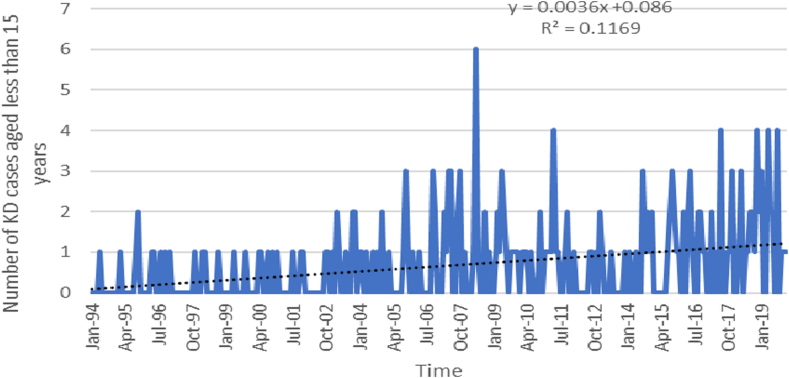
A trend analysis conducted from 1994 to 2019 revealed a statistically significant increase in KD cases among children aged <5, and <15 years. The analysis indicated a monthly rise of 0.002 (p-value < 0.0001) cases per 100,000 children aged <5 years, and 0.004 (p-value < 0.0001) cases per 100,000 children aged <15 years (Figs. 4 and 5).
Fig. 4.
Trend analysis of Kawasaki disease (KD) cases aged <5 years (Jan 1994–Dec 2019).
Fig. 5.
Trend analysis of Kawasaki disease (KD) cases aged <15 years (Jan 1994–Dec 2019).
Comparison of incidence of RF and KD at Chandigarh
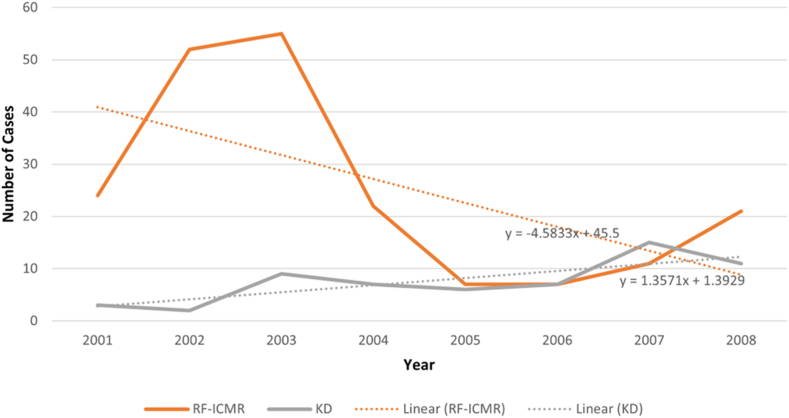
The incidence of RF and KD was compared using yearly data obtained during 2001–2008. Trend analysis showed an increase of 1.357 KD cases per year while RF cases were found to decline by 4.583 cases annually (Fig. 6). Further, we predict that the rise in incidence of KD is likely to continue for several years.
Fig. 6.
Difference in trends between Kawasaki disease (KD) cases and acute rheumatic fever (RF). Changes of Kawasaki disease cases in Chandigarh versus acute rheumatic fever national level estimates of disease generated by Indian Council of Medical Research (ICMR) during (2001–2008).
Discussion
In this study, we demonstrated that the incidence rates of KD in Chandigarh during 2015–2019 in children aged <5 years varied from 5.64/100,000 in 2015 to 9.72/100,000 in 2019. Corresponding incidence rates in children aged <15 years ranged from 2.65/100,000 in 2015 to 4.74/100,000 in 2019. The trend analysis indicated a monthly rise of 0.002 cases per 100,000 children aged <5 years, and 0.0165 cases per 100,000 children aged <15 years. CAAs were seen in 16.9% of patients during the acute stage and 7.2% at 6 weeks.
The three countries that report the highest incidence of KD are Japan, South Korea, and Taiwan.3,4,7,8 In these countries, the incidence is high and has continued to increase over the last three decades. Approximately 17,000 new KD cases were reported yearly in Japan before the COVID-19 pandemic.6
The first case of KD in Chandigarh was diagnosed in 1994.13 A robust hospital-based registry of children with KD has been maintained in our unit. The diagnosis of KD was based on AHA criteria. Management of KD was based on standard treatment protocols.1,2 There has been a consistent increase in the number of patients with KD at our centre over the last 26 years.13,16 (Fig. 4, Fig. 5) During 2015–2019, we diagnosed 539 children with KD at our centre. Of these, 83 (15.4%) were residents of Chandigarh. In comparison, the number of children diagnosed during 2009–2014 was 54.16 There has been a steadily increasing KD incidence from 2015 (5.64/100,000) to 2019 (9.72/100,000) in children aged <5 years. Corresponding incidence figures in children aged <15 years ranged from 2.65/100,000 in 2015 to 4.74/100,000 in 2019. Our incidence figures are comparable to the KD incidence reported from Europe (4.9–9/10,000 children <5 years).23, 24, 25 However, while we have been observing a steadily increasing incidence of KD at Chandigarh, the incidence appears to have plateaued in European countries.26 Interestingly, the reported incidence of KD in Chandigarh is lower than figures from other Asian countries (Japan, Taiwan, and South Korea).8,12 This may reflect the under-recognition of KD when it occurs in infancy and early childhood. Atypical and incomplete forms of KD are more common in these age groups. These presentations of KD often resemble symptoms of viral and bacterial infections, which can lead to confusion, especially among paediatricians in LMICs. Typically, the threshold for diagnosing a presumed infection is much lower than that for KD in these settings. As a result, many children with KD may remain undiagnosed.
In the present study, the median age at diagnosis of patients was 4 years, compared to 7 years during 1994–2008 and 3.5 years during 2009–2014.16 This shift in the age of diagnosis is probably due to an increase in awareness of KD amongst healthcare professionals in the region –resulting in a greater number of infants and young children getting diagnosed.7,16 However, the median age at diagnosis of KD at Chandigarh was still higher than that reported in Japan and countries in western part of the world.4,9,12 Genetic differences and under recognition of KD in younger children in our country may be the possible explanation for these differences.12 With increasing awareness of the disease over the last 26 years, the median age of diagnosis has decreased at our centre.
Seasonal variation in the incidence of KD in Chandigarh is not very clear. We noted an increased number of cases in Quarter 2 and Quarter 4. These quarters have the driest months of the year in Chandigarh and like the previous studies conducted in this institute, there is a surge of cases of KD during these months (Fig. 3, Supplementary Table S2).27 However, as the numbers are small, it is difficult to draw firm conclusions in this regard.
The occurrence of CAAs in the present study is higher than in the previous reports from our centre. This is likely due to better screening and imaging facilities that have progressively evolved at our centre.18,19,28,29 We started performing a Z-score-based assessments of coronary arteries in children with KD in 2013. Before that, we had been using absolute diameters for the assessment of CAAs. As Z score-based assessment is more sensitive, this may also contribute to the apparent increase in the detection of CAAs in the present study when compared to our previous studies.30,31 A population-based survey from the United Kingdom and Ireland has shown that the incidence of cardiac complications in the first month of illness was as high as 28%, while CAAs at 6 weeks of illness were reported in 19% of patients despite treatment. Further, CAAs were noted in 39% of children aged <1 year.32
In the developed world, KD has supplanted RF to emerge as the leading cause of acquired heart disease in children.1 Incidence of RF has decreased significantly and continues to show a declining trend in India as well.33,34 Therefore, we have juxtaposed the incidence of KD at Chandigarh with the estimated incidence of RF at the country level owing to lack of any epidemiological data on incidence of RF in this region. Trend analysis showed an increase of 1.357 KD cases per year while RF cases were found to decline by 4.583 cases annually (Fig. 6). To the best of our knowledge, in the last 15 years, there has been no reliable data on the incidence of RF in India. The last study on incidence rates of RF was published in 2013 and pertains to data collected from 2007 to 2008. In this study, the authors note that they came across no new cases of RF.35 Most other studies pertain to the prevalence of rheumatic heart disease (RHD), rather than the incidence of RF. It would be imprudent to compare the incidence of KD with the prevalence of RHD as the two metrics refer to different paradigms. However, the incidence of KD has continued to increase over the last two decades in several Asian countries like India, Japan, South Korea, and Taiwan. On the other hand, the incidence of RF has continued to show a steady decline.35, 36, 37 It appears, therefore, that KD is now likely to be the most important cause of paediatric acquired heart disease in children at Chandigarh. This may also be true for the country at large because most children with KD in India are not getting diagnosed and treated.10 Untreated KD may result in development of CAAs in 15–25% of children suffering from KD. This subgroup would be predisposed to the development of acute coronary syndrome later in life.
We acknowledge some of the inherent limitations of our study. First, it is possible that some cases of KD were managed elsewhere in Chandigarh during the acute phase without being referred to our centre. However, it is important to note that almost all children diagnosed with KD in the city get referred to our centre as we are the only tertiary care advanced facility in the city. Moreover, our centre follows the largest cohort of KD patients in the country.13,16 Second, there is a limitation pertaining to the method used for estimating the population growth rate for the intervening years between 2011 and 2019. As the Census data are not available yearly, we relied on projections made on decadal growth rates (17.19%) reported for Chandigarh to calculate the incidence rates from 2015 to 2019. While it is entirely possible that the growth rates for children aged <5 and <15 years may differ from the overall growth rates to the best of our knowledge there are no other available methods on which to base our calculations. Furthermore, it is not easy to compare the incidence of KD with that of RF because KD usually affects children aged <5 years, while RF occurs in children aged 5–15 years. Unfortunately, there is a paucity of data on the incidence of RF in the community. Most studies on the subject refer only to the prevalence of RHD.
To summarise, incidence rates of KD in Chandigarh during 2015–2019 in children aged <5 years varied from 5.64/100,000 in 2015 to 9.72/100,000 in 2019. Corresponding incidence rates in children aged <15 years ranged from 2.65/100,000 in 2015 to 4.74/100,000 in 2019. The trend analysis indicated a monthly rise of 0.002 cases per 100,000 children aged <5 years, and 0.0165 cases per 100,000 children aged <15 years. Cardiovascular complication rates in the acute stage were 16.9%, while CAAs at 6 weeks of illness were reported in 7.2% of patients despite therapy. With increased awareness amongst paediatricians, KD is now being diagnosed more frequently in infants and young children and is emerging as the leading cause of paediatric acquired heart disease at Chandigarh.
Contributors
RKP: Conceptualisation, writing of the initial draft, critical revision of the manuscript and final approval, patient management.
SB: Writing of the initial draft, revision of the manuscript, and patient management.
JD, RK, AKJ, AT, TK, PV: Writing and editing of the manuscript.
RN, JD, SP: Statistical analysis, writing, and editing of the manuscript.
PV, DS: Revision of the manuscript and patient management.
SN, MS, AR: Laboratory investigations, radiological investigations, writing and editing of the manuscript.
SS: Conceptualisation, critical revision of the manuscript, patient management.
Data sharing statement
The data underlying this article are available in the manuscript, in online Supplementary materials and in our records. These data can be shared on request.
Declaration of interests
All authors declare that they have no potential conflicts of interest.
Acknowledgements
None.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lansea.2024.100474.
Appendix A. Supplementary data
Supplementary Fig. S1.
Supplementary Fig. 1: Decadal growth rate of population in Chandigarh, India (1951–2011).
References
- 1.McCrindle B.W., Rowley A.H., Newburger J.W., et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American heart association. Circulation. 2017;135(17):e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 2.Newburger J.W., Takahashi M., Gerber M.A., et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the committee on rheumatic fever, endocarditis and Kawasaki disease, Council on cardiovascular disease in the young, American heart association. Circulation. 2004;110(17):2747–2771. doi: 10.1161/01.CIR.0000145143.19711.1E. [DOI] [PubMed] [Google Scholar]
- 3.Singh S., Vignesh P., Burgner D. The epidemiology of Kawasaki disease: a global update. Arch Dis Child. 2015;100(11):1084–1088. doi: 10.1136/archdischild-2015-308665. [DOI] [PubMed] [Google Scholar]
- 4.Lin M.T., Wu M.H. The global epidemiology of Kawasaki disease: review and future perspectives. Glob Cardiol Sci Pract. 2017;2017(3) doi: 10.21542/gcsp.2017.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iio K., Matsubara K., Miyakoshi C., et al. Incidence of Kawasaki disease before and during the COVID-19 pandemic: a retrospective cohort study in Japan. BMJ Paediatr Open. 2021;5(1) doi: 10.1136/bmjpo-2021-001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ae R., Makino N., Kuwabara M., et al. Incidence of Kawasaki disease before and after the COVID-19 pandemic in Japan. JAMA Pediatr. 2022;176(12):1217–1224. doi: 10.1001/jamapediatrics.2022.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang Y.H., Lin K.M., Ho S.C., Yan J.H., Lo M.H., Kuo H.C. Increased incidence of Kawasaki disease in taiwan in recent years: a 15 Years nationwide population-based cohort study. Front Pediatr. 2019;7:121. doi: 10.3389/fped.2019.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim G.B. Reality of Kawasaki disease epidemiology. Korean J Pediatr. 2019;62(8):292–296. doi: 10.3345/kjp.2019.62.8.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manlhiot C., Mueller B., O’Shea S., et al. Environmental epidemiology of Kawasaki disease: linking disease etiology, pathogenesis and global distribution. PLoS One. 2018;13(2) doi: 10.1371/journal.pone.0191087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiao F., Jindal A.K., Pandiarajan V., et al. The emergence of Kawasaki disease in India and China. Glob Cardiol Sci Pract. 2017;2017(3) doi: 10.21542/gcsp.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uehara R., Belay E.D. Epidemiology of Kawasaki disease in Asia, Europe, and the United States. J Epidemiol. 2012;22(2):79–85. doi: 10.2188/jea.JE20110103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elakabawi K., Lin J., Jiao F., Guo N., Yuan Z. Kawasaki disease: global burden and genetic background. Cardiol Res. 2020;11(1):9–14. doi: 10.14740/cr1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh S., Aulakh R., Bhalla A.K., et al. Is Kawasaki disease incidence rising in Chandigarh, north India? Arch Dis Child. 2011;96(2):137–140. doi: 10.1136/adc.2010.187380. [DOI] [PubMed] [Google Scholar]
- 14.Bhattad S., Gupta S., Israni N., Mohanty S. Profile of Kawasaki disease at a tertiary care center in India. Ann Pediatr Cardiol. 2021;14(2):187–191. doi: 10.4103/apc.apc_51_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jindal A.K., Pilania R.K., Guleria S., et al. Kawasaki disease in children older than 10 years: a clinical experience from northwest India. Front Pediatr. 2020;8:24. doi: 10.3389/fped.2020.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh S., Bhattad S. Kawasaki disease incidence at Chandigarh, north India, during 2009- 2014. Rheumatol Int. 2016;36(10):1391–1397. doi: 10.1007/s00296-016-3578-0. [DOI] [PubMed] [Google Scholar]
- 17.General information | Chandigarh, the official website of the Chandigarh Administration. https://chandigarh.gov.in/know-Chandigarh/general-information Available from:
- 18.Dallaire F., Dahdah N. New equations and a critical appraisal of coronary artery Z scores in healthy children. J Am Soc Echocardiogr. 2011;24(1):60–74. doi: 10.1016/j.echo.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 19.Singhal M., Singh S., Gupta P., Sharma A., Khandelwal N., Burns J.C. Computed tomography coronary angiography for evaluation of children with Kawasaki disease. Curr Probl Diagn Radiol. 2018;47(4):238–244. doi: 10.1067/j.cpradiol.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Singhal M., Pilania R.K., Jindal A.K., et al. Distal coronary artery abnormalities in Kawasaki disease: experience on CT coronary angiography in 176 children. Rheumatol Oxf Engl. 2023;62(2):815–823. doi: 10.1093/rheumatology/kead037. [DOI] [PubMed] [Google Scholar]
- 21.Chandigarh population sex ratio in Chandigarh literacy rate data 2011-2020. https://www.census2011.co.in/census/state/chandigarh.html Available from:
- 22.Kumar R., Sharma M. Community control of rheumatic fever/rheumatic heart disease in India. Comprehensive project report 2000-2010. https://main.icmr.nic.in/sites/default/files/reports/Jai%20Vigyan%20Mission%20Mode%20Project%20on%20Rheumatic%20Fever%20and%20Rheumatic%20Heart%20Disease%20%281%29.pdf Available from:
- 23.Fischer T.K., Holman R.C., Yorita K.L., Belay E.D., Melbye M., Koch A. Kawasaki syndrome in Denmark. Pediatr Infect Dis J. 2007;26(5):411–415. doi: 10.1097/01.inf.0000263742.74634.70. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez-Manubens J., Anton J., Prada F., et al. Incidence and clinical features of Kawasaki disease in Catalonia (Spain) Pediatr Rheumatol. 2014;12(1) doi: 10.1186/1546-0096-12-S1-P123. [DOI] [PubMed] [Google Scholar]
- 25.Heuclin T., Dubos F., Hue V., et al. Increased detection rate of Kawasaki disease using new diagnostic algorithm, including early use of echocardiography. J Pediatr. 2009;155(5):695–699.e1. doi: 10.1016/j.jpeds.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 26.Piram M. Epidemiology of Kawasaki disease in Europe. Front Pediatr. 2021;9 doi: 10.3389/fped.2021.673554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meteorological centre Chandigarh.pdf. https://mausam.imd.gov.in/imd_latest/contents/pdf/pubbrochures/Meteorological%20Centre%20Chandigarh.pdf [cited 2024 May 24]. Available from:
- 28.Singhal M., Pilania R.K., Gupta P., Johnson N., Singh S. Emerging role of computed tomography coronary angiography in evaluation of children with Kawasaki disease. World J Clin Pediatr. 2023;12(3):97–106. doi: 10.5409/wjcp.v12.i3.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singhal M., Pilania R.K., Thangaraj A., et al. The value of CT coronary angiography for a comprehensive assessment of left circumflex artery in Kawasaki disease: 9 years of experience from a tertiary center. Lancet Reg Health Southeast Asia. 2024;29 doi: 10.1016/j.lansea.2024.100471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim S.H. Diagnosis of coronary artery abnormalities in Kawasaki disease: recent guidelines and z score systems. Clin Exp Pediatr. 2021;65(9):430–438. doi: 10.3345/kjp.2021.65.9.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson D.L., Ware A.L., Sauer M.C., et al. Implications of changing Z-score models for coronary artery dimensions in Kawasaki disease. Pediatr Cardiol. 2021;42(2):432–441. doi: 10.1007/s00246-020-02578-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tulloh R.M.R., Mayon-White R., Harnden A., et al. Kawasaki disease: a prospective population survey in the UK and Ireland from 2013 to 2015. Arch Dis Child. 2019;104(7):640–646. doi: 10.1136/archdischild-2018-315293. [DOI] [PubMed] [Google Scholar]
- 33.Negi P.C., Sondhi S., Asotra S., Mahajan K., Mehta A. Current status of rheumatic heart disease in India. Indian Heart J. 2019;71(1):85–90. doi: 10.1016/j.ihj.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar R.K., Tandon R. Rheumatic fever & rheumatic heart disease: the last 50 years. Indian J Med Res. 2013;137(4):643–658. doi: 10.4103/0971-5916.65504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Negi P.C., Kanwar A., Chauhan R., Asotra S., Thakur J.S., Bhardwaj A.K. Epidemiological trends of RF/RHD in school children of Shimla in north India. Indian J Med Res. 2013;137(6):1121–1127. doi: 10.4103/0971-5916.137609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar R.K., Antunes M.J., Beaton A., et al. Contemporary diagnosis and management of rheumatic heart disease: implications for closing the gap: a scientific statement from the American heart association. Circulation. 2020;142(20):e337–e357. doi: 10.1161/CIR.0000000000000913. [DOI] [PubMed] [Google Scholar]
- 37.Thakur J.S., Negi P.C., Ahluwalia S.K., Vaidya N.K. Epidemiological survey of rheumatic heart disease among school children in the Shimla Hills of northern India: prevalence and risk factors. J Epidemiol Community Health. 1996;50(1):62–67. doi: 10.1136/jech.50.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.