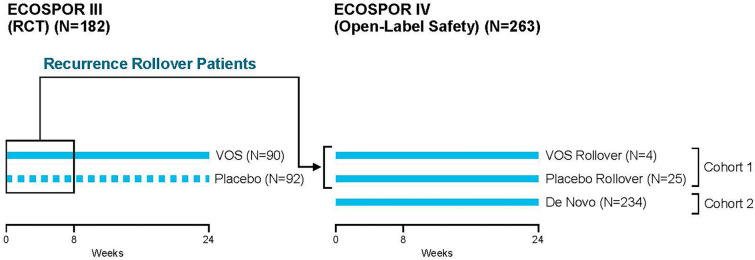
Fig. 1.
Phase 3 clinical studies included in the integrated analysis of VOS safety and efficacy. RCT randomized controlled trial. Patients in ECOSPOR III who experienced CDI recurrence prior to 8 weeks post-treatment (indicated by rectangular box) were eligible to rollover into ECOSPOR IV. The same VOS treatment regimen (3 × 107 spore colony forming units [SCFUs] in four capsules once daily for three consecutive days) was administered in both ECOSPOR III and ECOSPOR IV. Blue solid lines indicate data included in the integrated datasets. Placebo data (dashed blue lines) were not included in the integrated datasets. Integrated data was available from 349 unique patients treated with at least one VOS dosing regimen (90 patients from ECOSPOR III [note that 4 of these patients received a second regimen in ECOSPOR IV], 25 placebo rollover patients in ECOSPOR IV, and 234 de novo patients in ECOSPOR IV