Abstract
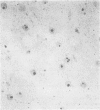
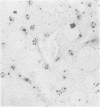
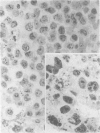
A series of 54 liver biopsy specimens was studied by means of the argyrophil (AgNOR) technique for nucleolar organiser region (NOR)-associated proteins. These included normal livers and livers affected by chronic active hepatitis, cirrhosis, hepatocellular carcinoma and adenoma. Four of the cases of cirrhosis showed liver cell dysplasia. The mean numbers of NOR sites in normal, cirrhotic, and carcinomatous livers were significantly different: adenoma had similar mean counts to those in chronic active hepatitis (CAH). There was no overlap between the ranges of NOR counts in normal, cirrhotic, and malignant liver specimens. Where cirrhosis and hepatocellular carcinoma were present in the same specimen, the AgNOR counts were higher in the carcinomatous than cirrhotic areas. To investigate the prospective value of the method a further seven biopsy specimens were studied; in these it had not been possible to decide on a diagnosis between normality and cirrhosis or cirrhosis and hepatocellular carcinoma. In all seven specimens a repeat biopsy or necropsy gave results as predicted by AgNOR staining. It is therefore proposed that quantitation of staining for NOR-associated proteins is a diagnostically useful method in liver disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayres J. G., Crocker J. G., Skilbeck N. Q. Differentiation of malignant from normal and reactive mesothelial cells by the argyrophil technique for nucleolar organiser region associated proteins. Thorax. 1988 May;43(5):366–370. doi: 10.1136/thx.43.5.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch H., Daskal Y., Gyorkey F., Smetana K. Silver staining of nucleolar granules in tumor cells. Cancer Res. 1979 Mar;39(3):857–863. [PubMed] [Google Scholar]
- Crocker J., Ayres J., McGovern J. Nucleolar organiser regions in small cell carcinoma of the bronchus. Thorax. 1987 Dec;42(12):972–975. doi: 10.1136/thx.42.12.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker J., Macartney J. C., Smith P. J. Correlation between DNA flow cytometric and nucleolar organizer region data in non-Hodgkin's lymphomas. J Pathol. 1988 Feb;154(2):151–156. doi: 10.1002/path.1711540207. [DOI] [PubMed] [Google Scholar]
- Crocker J., Skilbeck N. Nucleolar organiser region associated proteins in cutaneous melanotic lesions: a quantitative study. J Clin Pathol. 1987 Aug;40(8):885–889. doi: 10.1136/jcp.40.8.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossen P. E., Godwin J. M. Rearrangement and possible amplification of the ribosomal RNA gene sites in the human chronic myelogenous leukemia cell line K562. Cancer Genet Cytogenet. 1985 Sep;18(1):27–30. doi: 10.1016/0165-4608(85)90035-4. [DOI] [PubMed] [Google Scholar]
- Egan M. J., Crocker J. Nucleolar organizer regions in cutaneous tumours. J Pathol. 1988 Mar;154(3):247–253. doi: 10.1002/path.1711540307. [DOI] [PubMed] [Google Scholar]
- Egan M. J., Raafat F., Crocker J., Smith K. Nucleolar organiser regions in fibrous proliferations of childhood and infantile fibrosarcoma. J Clin Pathol. 1988 Jan;41(1):31–33. doi: 10.1136/jcp.41.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan M. J., Raafat F., Crocker J., Smith K. Nucleolar organizer regions in small cell tumours of childhood. J Pathol. 1987 Nov;153(3):275–280. doi: 10.1002/path.1711530312. [DOI] [PubMed] [Google Scholar]
- Elliott C. J., Sloane J. P., Pallett C. D., Sanderson K. V. Cutaneous leucocyte composition after human allogeneic bone marrow transplantation: relationship to marrow purging, histology and clinical rash. Histopathology. 1988 Jan;12(1):1–16. doi: 10.1111/j.1365-2559.1988.tb01912.x. [DOI] [PubMed] [Google Scholar]
- Field D. H., Fitzgerald P. H., Sin F. Y. Nucleolar silver-staining patterns related to cell cycle phase and cell generation of PHA-stimulated human lymphocytes. Cytobios. 1984;41(161):23–33. [PubMed] [Google Scholar]
- Hall P. A., Crocker J., Watts A., Stansfeld A. G. A comparison of nucleolar organizer region staining and Ki-67 immunostaining in non-Hodgkin's lymphoma. Histopathology. 1988 Apr;12(4):373–381. doi: 10.1111/j.1365-2559.1988.tb01952.x. [DOI] [PubMed] [Google Scholar]
- Henry M. A., Tange J. D. Lesions of the renal papilla induced by paracetamol. J Pathol. 1987 Jan;151(1):11–19. doi: 10.1002/path.1711510103. [DOI] [PubMed] [Google Scholar]
- Reeves B. R., Casey G., Honeycombe J. R., Smith S. Correlation of differentiation state and silver staining of nucleolar organizers in the promyelocytic leukemia cell line HL-60. Cancer Genet Cytogenet. 1984 Oct;13(2):159–166. doi: 10.1016/0165-4608(84)90057-8. [DOI] [PubMed] [Google Scholar]
- Walker R. A. The histopathological evaluation of nucleolar organizer region proteins. Histopathology. 1988 Feb;12(2):221–223. doi: 10.1111/j.1365-2559.1988.tb01932.x. [DOI] [PubMed] [Google Scholar]
- de Capoa A., Baldini A., Marlekaj P., Natoli C., Rocchi M., Archidiacono N., Cianfarani S., Spadoni G. L., Boscherini B. Hormone-modulated rRNA gene activity is visualized by selective staining of the NOs. Cell Biol Int Rep. 1985 Sep;9(9):791–796. doi: 10.1016/0309-1651(85)90097-9. [DOI] [PubMed] [Google Scholar]