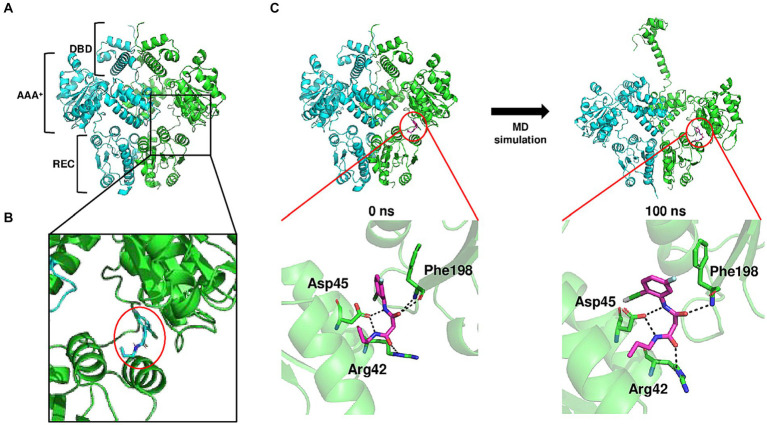
Figure 4.
Conformational change of BrpR upon the interaction with BFstatin. (A) Molecular structure of BrpR dimer was predicted using the AlphaFold2 algorithm. DBD, DNA-binding domain; AAA+, ATPase associated with diverse cellular activities; REC, receiver domain. (B) Molecular docking of the BrpR dimer and BFstatin was performed using AutoDock Vina. The ligand-binding pocket of BrpR and docked BFstatin is presented in a close-up view. BFstatin is inside the red circle. (C) MD simulation with the docked BrpR-BFstatin complex was carried out using Gromacs software. The structure of the BrpR-BFstatin complex and close-up views of the ligand-binding pocket are presented vertically. Left, BrpR-BFstatin complex before MD simulation; Right, BrpR-BFstatin complex after 100 ns MD simulation.