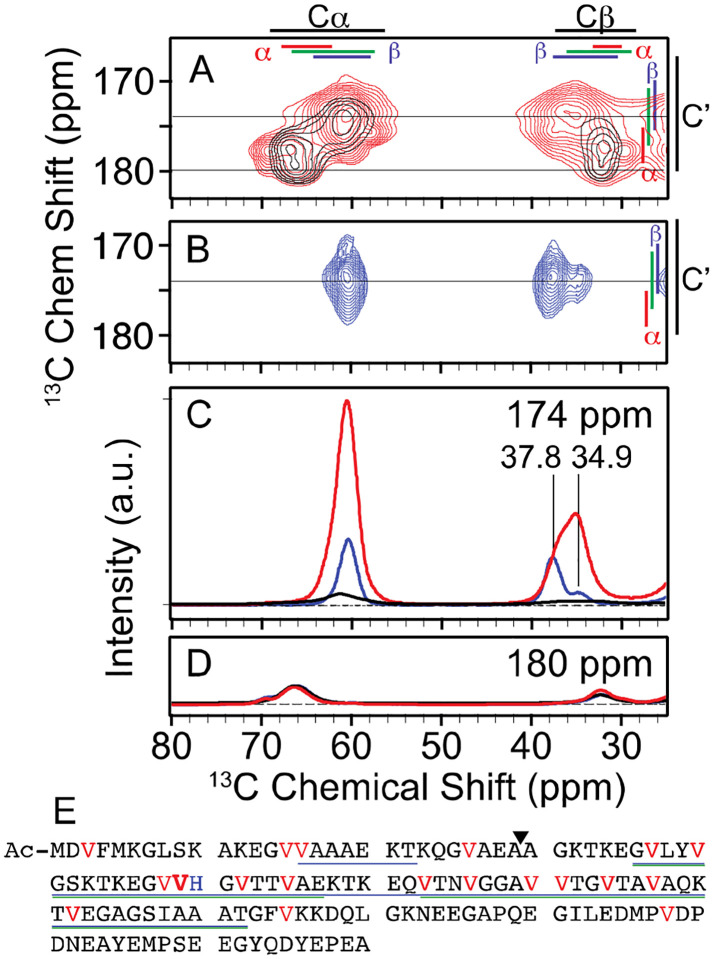
Figure 1:
A) Overlay of 2D 13C–13C DARR spectra of uniformly 13C-valine labeled Ac-α-syn(A53T) fibrils (red) and segmentally 13C-valine labeled fibrils where only the four N terminal valines (black) are labeled. Average chemical shifts +/− two standard deviation are marked for each atom (black) as well as for the α-helical (red), random coil (green), and β (blue) secondary structural elements. B) NCOCx spectra of uniformly 13C-valine and 15N-histidine labeled fibrils that is specific for V49 (blue). One-dimensional slice from the 2D spectra at C) 174 ppm and D) 180 ppm. The intensity of the V49 spectrum (blue) is displayed at 10 times the relative intensity for visibility and the peak centers are indicated. NMR spectra were recorded at a field strength of 14 T (600 MHz) and MAS frequencies of 12 kHz at 104 K. NMR samples were cryoprotected with 15% d8-12C-glycerol and doped with 7 mM AMUPol. E) Primary sequence of Ac-α-syn(A53T). The 19 valines (highlighted in red) are distributed throughout the sequence, with V49 bolded for emphasis. H50 is colored blue. Regions visible in the cryo-EM density for polymorph A (blue) and polymorph B (green) are underlined. The ligation site for segmental isotopic labeling is indicated by a triangle.