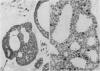Abstract
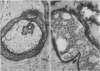
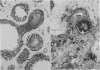
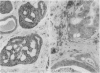
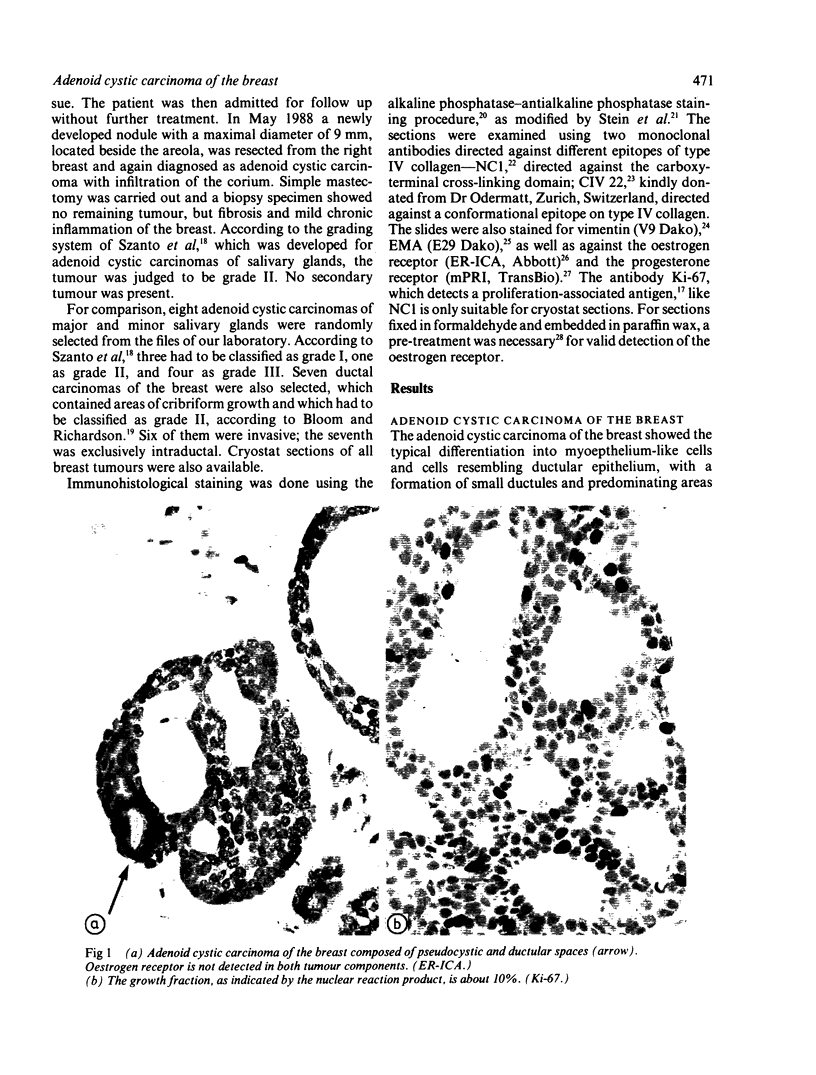
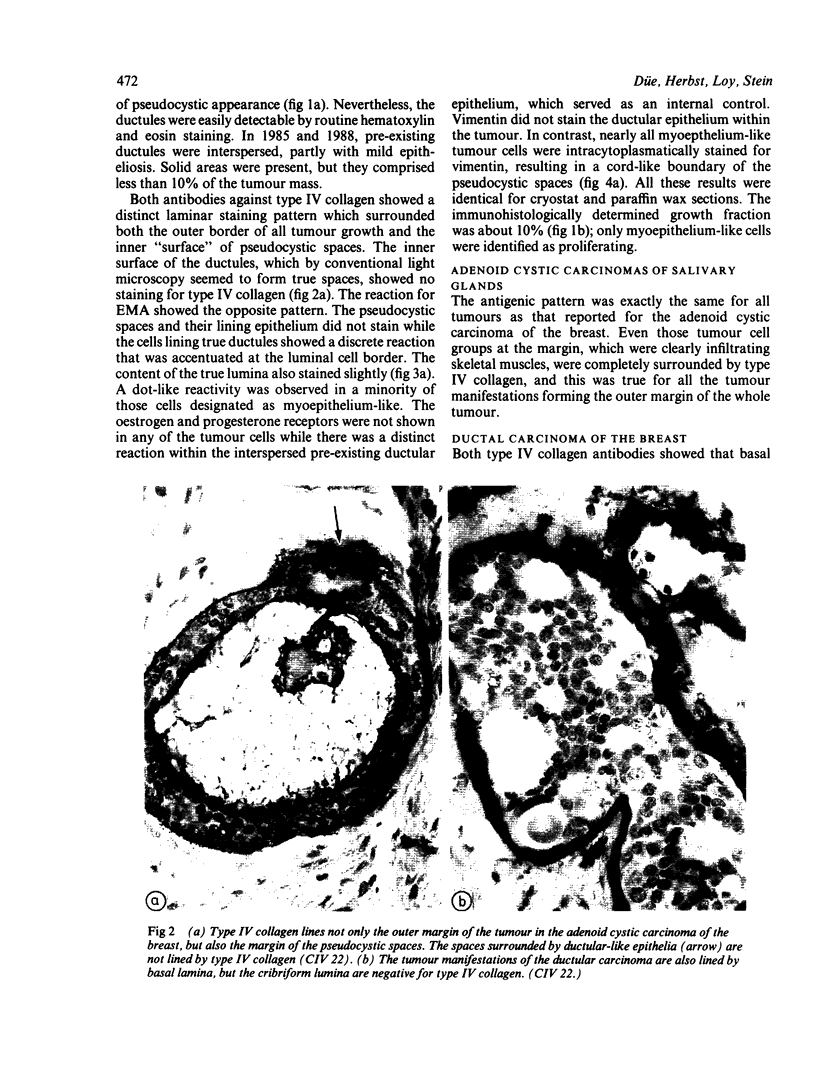
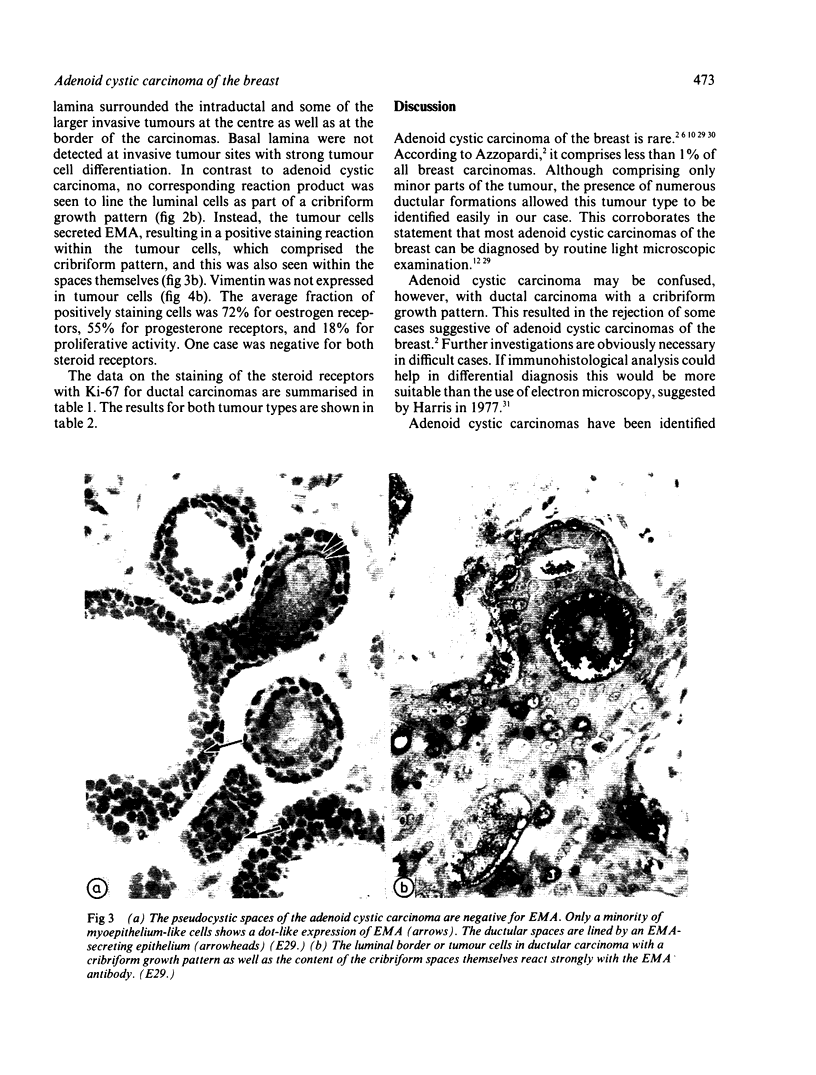
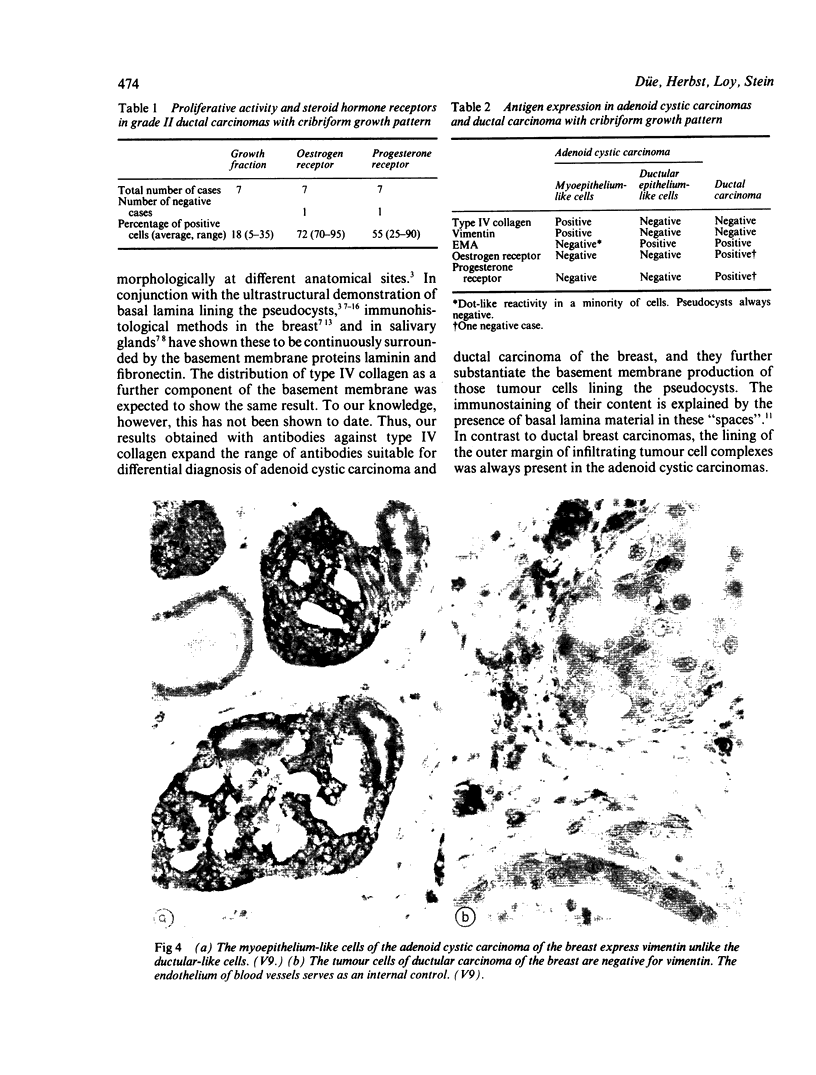
An adenoid cystic carcinoma of the breast in a 78 year old woman was analysed immunohistologically for the production of type IV collagen, the expression of vimentin, epithelial membrane antigen (EMA) and steroid receptors, and the proliferative activity of the tumour cells. The data were compared with those obtained in eight adenoid cystic carcinomas of salivary glands and in ductal carcinomas of the breast with a cribriform growth pattern. The patients' ages were as follows: 45-80 years (mean 63.2) for the salivary gland carcinomas; 37-69 years (mean 50.6) for the ductal breast carcinomas. In contrast to the cribriform spaces of ductal carcinomas, the pseudocysts in adenoid cystic carcinomas were lined by type IV collagen. The opposite pattern was observed for EMA. Like the myoepithelium of normal breast, the myoepithelium-like cells of adenoid cystic carcinoma stained positive for vimentin while the ductular epithelium-like ones did not. All adenoid cystic carcinomas, including that of the breast, were negative for the oestrogen and progesterone receptors, unlike the ductal carcinomas. Proliferative activity of the adenoid cystic carcinoma of the breast was relatively low. These data broaden the range of antibodies suitable for differential diagnosis of both tumour types. They may explain the differences in prognosis, and they explain why hormonal treatment or radiotherapy of adenoid cystic carcinoma of the breast are often ineffectual.
Full text
PDF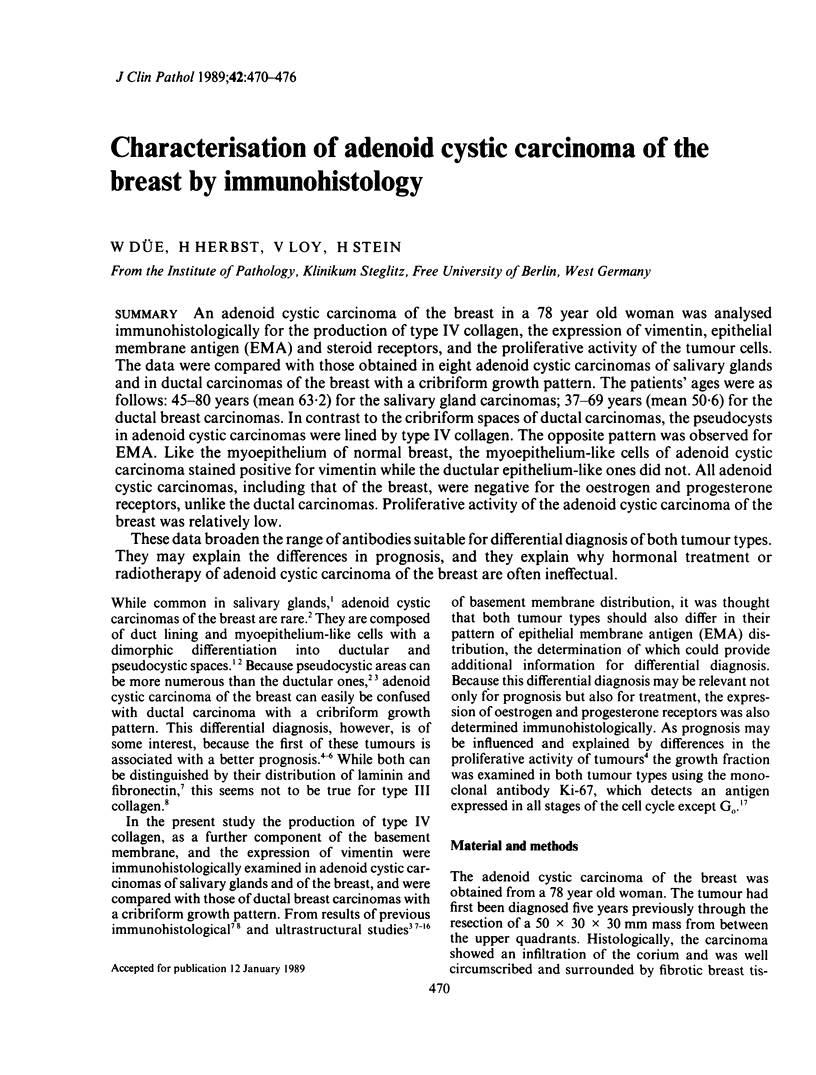


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLOOM H. J., RICHARDSON W. W. Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer. 1957 Sep;11(3):359–377. doi: 10.1038/bjc.1957.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard N. J., Hall P. A., Lemoine N. R., Kadar N. Proliferative index in breast carcinoma determined in situ by Ki67 immunostaining and its relationship to clinical and pathological variables. J Pathol. 1987 Aug;152(4):287–295. doi: 10.1002/path.1711520407. [DOI] [PubMed] [Google Scholar]
- Blackshaw A. J., Levison D. A. Eosinophilic infiltrates of the gastrointestinal tract. J Clin Pathol. 1986 Jan;39(1):1–7. doi: 10.1136/jcp.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boss W. F., Morré D. J., Mollenhauer H. H. Monensin-induced swelling of Golgi apparatus cisternae mediated by a proton gradient. Eur J Cell Biol. 1984 May;34(1):1–8. [PubMed] [Google Scholar]
- Cavanzo F. J., Taylor H. B. Adenoid cystic carcinoma of the breast. An analysis of 21 cases. Cancer. 1969 Oct;24(4):740–745. doi: 10.1002/1097-0142(196910)24:4<740::aid-cncr2820240412>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Ceriani R. L., Thompson K., Peterson J. A., Abraham S. Surface differentiation antigens of human mammary epithelial cells carried on the human milk fat globule. Proc Natl Acad Sci U S A. 1977 Feb;74(2):582–586. doi: 10.1073/pnas.74.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- D'Ardenne A. J., Burns J., Sykes B. C., Bennett M. K. Fibronectin and type III collagen in epithelial neoplasms of gastrointestinal tract and salivary gland. J Clin Pathol. 1983 Jul;36(7):756–763. doi: 10.1136/jcp.36.7.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman B. A., Oberman H. A. Adenoid cystic carcinoma of the breast. Am J Clin Pathol. 1970 Jul;54(1):1–14. doi: 10.1093/ajcp/54.1.1. [DOI] [PubMed] [Google Scholar]
- Galloway J. R., Woolner L. B., Clagett O. T. Adenoid cystic carcinoma of the breast. Surg Gynecol Obstet. 1966 Jun;122(6):1289–1294. [PubMed] [Google Scholar]
- Gerdes J., Lemke H., Baisch H., Wacker H. H., Schwab U., Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984 Oct;133(4):1710–1715. [PubMed] [Google Scholar]
- Gerdes J., Pickartz H., Brotherton J., Hammerstein J., Weitzel H., Stein H. Growth fractions and estrogen receptors in human breast cancers as determined in situ with monoclonal antibodies. Am J Pathol. 1987 Dec;129(3):486–492. [PMC free article] [PubMed] [Google Scholar]
- Gould V. E., Miller J., Jao W. Ultrastructure of medullary, intraductal, tubular and adenocystic breast carcinomas: comparative patterns of myoepithelial differentiation and basal lamina deposition. Am J Pathol. 1975 Mar;78(3):401–408. [PMC free article] [PubMed] [Google Scholar]
- Gusterson B. A., Warburton M. J., Mitchell D., Ellison M., Neville A. M., Rudland P. S. Distribution of myoepithelial cells and basement membrane proteins in the normal breast and in benign and malignant breast diseases. Cancer Res. 1982 Nov;42(11):4763–4770. [PubMed] [Google Scholar]
- Harris M. Pseudoadenoid cystic carcinoma of the breast. Arch Pathol Lab Med. 1977 Jun;101(6):307–309. [PubMed] [Google Scholar]
- Kern W. H. Morphologic and clinical aspects of estrogen receptors in carcinoma of the breast. Surg Gynecol Obstet. 1979 Feb;148(2):240–242. [PubMed] [Google Scholar]
- Lawrence J. B., Mazur M. T. Adenoid cystic carcinoma: a comparative pathologic study of tumors in salivary gland, breast, lung, and cervix. Hum Pathol. 1982 Oct;13(10):916–924. doi: 10.1016/s0046-8177(82)80052-x. [DOI] [PubMed] [Google Scholar]
- Odermatt B. F., Lang A. B., Rüttner J. R., Winterhalter K. H., Trüeb B. Monoclonal antibodies to human type IV collagen: useful reagents to demonstrate the heterotrimeric nature of the molecule. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7343–7347. doi: 10.1073/pnas.81.23.7343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orenstein J. M., Dardick I., van Nostrand A. W. Ultrastructural similarities of adenoid cystic carcinoma and pleomorphic adenoma. Histopathology. 1985 Jun;9(6):623–638. doi: 10.1111/j.1365-2559.1985.tb02843.x. [DOI] [PubMed] [Google Scholar]
- Perrot-Applanat M., Logeat F., Groyer-Picard M. T., Milgrom E. Immunocytochemical study of mammalian progesterone receptor using monoclonal antibodies. Endocrinology. 1985 Apr;116(4):1473–1484. doi: 10.1210/endo-116-4-1473. [DOI] [PubMed] [Google Scholar]
- Pickartz H., Gerdes J., Schwarting R., Stein H. Zur Bedeutung des lympho-phagozytären Infiltrates von Mammakarzinomen für die lymphonoduläre Metastasierung. Verh Dtsch Ges Pathol. 1985;69:269–273. [PubMed] [Google Scholar]
- Reiner A., Reiner G., Spona J., Schemper M., Kolb R., Jakesz R., Holzner J. H. Vergleich von immunhistochemischem und biochemischem Oestrogenrezeptornachweis beim Mammakarzinom und Beziehungen zur histologischen Tumorklassifikation. Verh Dtsch Ges Pathol. 1986;70:243–246. [PubMed] [Google Scholar]
- Ro J. Y., Silva E. G., Gallager H. S. Adenoid cystic carcinoma of the breast. Hum Pathol. 1987 Dec;18(12):1276–1281. doi: 10.1016/s0046-8177(87)80413-6. [DOI] [PubMed] [Google Scholar]
- Schuppan D., Besser M., Schwarting R., Hahn E. G. Radioimmunoassay for the carboxy-terminal cross-linking domain of type IV (basement membrane) procollagen in body fluids. Characterization and application to collagen type IV metabolism in fibrotic liver disease. J Clin Invest. 1986 Jul;78(1):241–248. doi: 10.1172/JCI112557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein H., Gatter K., Asbahr H., Mason D. Y. Use of freeze-dried paraffin-embedded sections for immunohistologic staining with monoclonal antibodies. Lab Invest. 1985 Jun;52(6):676–683. [PubMed] [Google Scholar]
- Sumpio B. E., Jennings T. A., Merino M. J., Sullivan P. D. Adenoid cystic carcinoma of the breast. Data from the Connecticut Tumor Registry and a review of the literature. Ann Surg. 1987 Mar;205(3):295–301. doi: 10.1097/00000658-198703000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szanto P. A., Luna M. A., Tortoledo M. E., White R. A. Histologic grading of adenoid cystic carcinoma of the salivary glands. Cancer. 1984 Sep 15;54(6):1062–1069. doi: 10.1002/1097-0142(19840915)54:6<1062::aid-cncr2820540622>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Tandler B. Ultrastructure of adenoid cystic carcinoma of salivary gland origin. Lab Invest. 1971 Jun;24(6):504–512. [PubMed] [Google Scholar]
- Tavassoli F. A., Norris H. J. Mammary adenoid cystic carcinoma with sebaceous differentiation. A morphologic study of the cell types. Arch Pathol Lab Med. 1986 Nov;110(11):1045–1053. [PubMed] [Google Scholar]
- Wells C. A., Nicoll S., Ferguson D. J. Adenoid cystic carcinoma of the breast: a case with axillary lymph node metastasis. Histopathology. 1986 Apr;10(4):415–424. doi: 10.1111/j.1365-2559.1986.tb02494.x. [DOI] [PubMed] [Google Scholar]
- Zaloudek C., Oertel Y. C., Orenstein J. M. Adenoid cystic carcinoma of the breast. Am J Clin Pathol. 1984 Mar;81(3):297–307. doi: 10.1093/ajcp/81.3.297. [DOI] [PubMed] [Google Scholar]