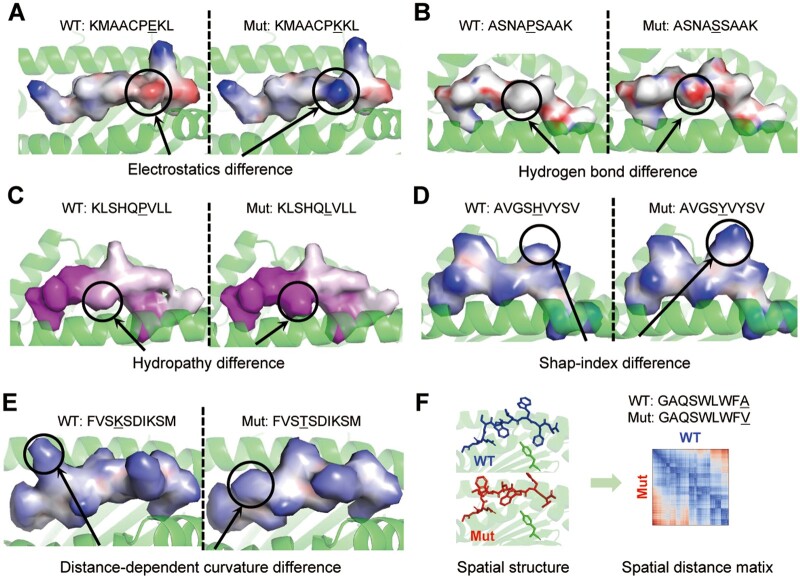
Figure 5.
Comparison of the surface and structural features between Mut and WT counterparts. (A) Electrostatic features between KDM5C E656K (positive charge) and WT (negative charge). (B) Hydrogen bond features between HERC1 P3278S (hydrogen bond donors) and WT. (C) Hydropathy features between SNX24 P132L (hydrophobic) and WT. (D) Shape-index features between PGM5 H469Y and WT. (E) Distance-dependent curvature features between OXSM K109T and WT. (F) Spatial structure and atom spatial distance matrix of TENM3 A2490V and WT.