Abstract
T cell engaging bispecific antibodies (TCBs) have recently become significant in cancer treatment. In this study we developed MSLN490, a novel TCB designed to target mesothelin (MSLN), a glycosylphosphatidylinositol (GPI)-linked glycoprotein highly expressed in various cancers, and evaluated its efficacy against solid tumors. CDR walking and phage display techniques were used to improve affinity of the parental antibody M912, resulting in a pool of antibodies with different affinities to MSLN. From this pool, various bispecific antibodies (BsAbs) were assembled. Notably, MSLN490 with its IgG-[L]-scFv structure displayed remarkable anti-tumor activity against MSLN-expressing tumors (EC50: 0.16 pM in HT-29-hMSLN cells). Furthermore, MSLN490 remained effective even in the presence of non-membrane-anchored MSLN (soluble MSLN). Moreover, the anti-tumor activity of MSLN490 was enhanced when combined with either Atezolizumab or TAA × CD28 BsAbs. Notably, a synergistic effect was observed between MSLN490 and paclitaxel, as paclitaxel disrupted the immunosuppressive microenvironment within solid tumors, enhancing immune cells infiltration and improved anti-tumor efficacy. Overall, MSLN490 exhibits robust anti-tumor activity, resilience to soluble MSLN interference, and enhanced anti-tumor effects when combined with other therapies, offering a promising future for the treatment of a variety of solid tumors. This study provides a strong foundation for further exploration of MSLN490’s clinical potential.

Keywords: solid tumors, T cell engaging bispecific antibodies, MSLN490, mesothelin, paclitaxel
Introduction
Over the past decade, there has been growing interest in therapeutic approaches redirecting T cells toward various types of cancer [1–3]. T cell-engaging bispecific antibodies (TCBs) have emerged as promising cancer therapeutics capable of recruiting and activating T cells expressing CD3 against tumor cells expressing tumor-associated antigens (TAAs) [4]. Notably, TCBs have demonstrated clinical success in hematologic malignancies. For instance, Mosunetuzumab (CD20 × CD3) [5], Epcoritamab (CD20 × CD3) [6], and Talquetamab (GPRC5D × CD3) [7] have recently received FDA approval for the treatment of hematologic malignancies. However, translating this success to solid tumors poses a significant challenge, influenced by several factors [8, 9]. Effective T cell infiltration into the tumor microenvironment and their subsequent interaction with tumor cells are critical determinants of therapeutic efficacy [10]. Yet, T cell trafficking and tumor infiltration may be impeded by various factors, such as limited chemokine expression or physical barriers within the tumor microenvironment [11]. Additionally, the number of infiltrating T cells may be insufficient to elicit a robust anti-tumor response, potentially limiting the therapeutic potential of TCBs in clinical applications.
The absence of tumor-specific antigens presents a significant challenge in the success of T cell-based immunotherapies for solid tumors. Therefore, identifying targeting tumor-restricted antigens is crucial for developing effective TCB treatments. Mesothelin (MSLN) is a glycosylphosphatidylinositol (GPI)-anchored membrane glycoprotein that exhibits high expression in various cancers, including pancreatic cancer, gastric cancer, ovarian cancer, mesothelioma, and other cancer types [12–15]. While the precise biological function of MSLN remains unclear, its upregulation in tumor cells has been observed as a negative prognostic factor in many cancer types, suggesting its potential involvement in tumor progression and metastasis [15]. Despite also being expressed on normal mesothelial cells, its primarily restricted distribution in normal tissues makes it a promising target for tumor-specific therapies [15, 16]. Human MSLN is initially synthesized as a 71-kDa precursor protein. After processing, the 31-kDa N-terminal domain is released as a soluble protein called megakaryocyte potentiating factor, whereas the 40-kDa C-terminal domain remains anchored to the plasma membrane, forming mature MSLN [17]. Furthermore, emerging evidence indicates that mesothelin, particularly its extracellular domain, can also be shed from tumor cells [18, 19]. The presence of non-membrane-anchored MSLN (soluble MSLN, sMSLN) may pose challenge for the action by the antibodies and antibody-based agents, such as CAR-T cells and TCB therapies, as they may encounter sMSLN before reaching and recognizing MSLN on tumor cells [20]. Research has shown that antibodies capable of specifically recognizing a peptide from the C-terminus of MSLN, which contains residues in the shedding site region, can avoid the impact of sMSLN [21, 22].
Since the inception of TCBs, a myriad of antibody formats have been developed [23]. However, it has become increasingly apparent that the development of safe and effective TCBs is a complex and nuanced process that requires careful consideration and optimization, as evidenced by the challenges encountered during the testing of various antibody formats [24–26]. In the early stages of our research, we employed CDR walking and phage display techniques to derive affinity-matured targeting sequences against MSLN from the parental antibody M912 [27]. From these sequences, we selected four candidates to investigate the impact of TCBs with varying affinities and structures on their anti-tumor efficacy.
Our study results revealed that MSLN490, with its IgG-[L]-scFv structure, displayed remarkable antitumor activity against MSLN-expressing tumors. In vitro, sMSLN had minimal impact on the MSLN490-mediated killing of MSLN-positive tumor cells by T cells, in comparison to MSLN530, which has a higher affinity for MSLN, and the positive control HPN536 [16]. Furthermore, combining MSLN490 with Atezolizumab or TAA × CD28 bispecific antibodies (BsAbs) enhanced anti-tumor responses. Our findings also suggested that combining MSLN490 with Paclitaxel held promise as a strategy to enhance its efficacy in noninflamed tumors.
Materials and methods
Cells and cell lines
The Raji (TCHu 44), OVCAR3 (TCHu228), SKOV3 (TCHu185), Capan-2 (TCHu259), AsPC-1 (SCSP-5080), and NCI-H292 (Tchu122) cell lines were acquired from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Additionally, the NCI-N87 (CL-0169) and MKN45 (CL-0292) cell lines were obtained from Procell Life Science & Technology (Wuhan, China). HT29-MSLN cells were generated as described previously [28]. All the above cell lines were cultured in RPMI-1640 (11875093, Thermo Fisher Scientific, Waltham, MA, USA) containing 10% FBS (10099141, Thermo Fisher Scientific), except for SKOV3, which was cultured in McCoy’s 5A (16600082, Thermo Fisher Scientific) containing 10% FBS. Cells were maintained in an incubator of 5% CO2 at 37 °C. Peripheral blood mononuclear cells (PBMCs) were obtained from SailyBio (Shanghai, China) for in vitro or in vivo studies.
Affinity maturation by phage display
We first analyzed M912 variable sequences [27] using IMGT/V-Quest online tool. The M912 heavy chain variable sequence (IGHV) was identical to human IGHV4-61*01, whereas the M912 light chain variable sequence (IGKV) was almost identical to human IGKV1-39*01 except one amino acid at position 76. We focused our affinity maturation primarily on HCDR3 and LCDR3. Additionally, we changed the non-germline residue in the M912 light chain (G76) to its germline counterpart arginine (R). The antibody gene sequences were randomly mutated using PCR amplification, and the resulting products were purified and ligated into the phage display vector pCANTAB6. After vector linearization, they were introduced into competent bacteria through electroporation and incubated in 2 × YT medium for 1 h. Helper phage was added, and the bacteria were cultured overnight at 30 °C with shaking at 250 rpm. Following centrifugation, the precipitated phage represented the antibody mutant library. Subsequent antibody discovery procedures were employed to screen for our optimized target antibodies from this library.
Protein sequences
The F405L mutation was introduced in the Fc region of all MSLN monoclonal antibodies (mAbs) and Tissue factor (TF) mAb [29]. The K409R mutation was introduced in CD3 (OKT3) and CD28 mAbs (WO 2020/198009 A1), and simultaneously incorporated the P329G, L234A, L235A mutation to eliminate the antibody-dependent cell-mediated cytotoxicity (ADCC) effect. When constructing the “2 + 1” type TCB, the knobs-into-holes technology was employed. The scFv fragment of LP2/HP4-44 and anti-CD3 were fused with the hole variant Fc region, while the scFv fragment of LP2/HP4-44 alone was fused with the knob variant Fc region. Additionally, P329G, L234A, L235A mutations were introduced to eliminate the ADCC effect as well. M912 (IgG-[L]-scFv), HP1-A13 (IgG-[L]-scFv), MSLN490, and MSLN530 were constructed based on the IgG-[L]-scFv structure introduced by the Memorial Sloan Kettering Cancer Center [26]. However, optimizations were performed to the linker in these constructs. HPN536, a TCB targeting MSLN, served as a positive control and the sequences were described in the patent WO 2018/209304 Al. The sequences of REGN7075 were described in the patent WO 2020/198009 A1.
Protein production
All proteins were expressed using the Expi293 cell line (A14527, Thermo Fisher Scientific). Briefly, expression plasmids for each antibody were amplified and purified using the Endo-Free Plasmid Maxi Kit (D6926-03, Omega Bio-tek, Norcross, GA, USA). Purified plasmid DNA was transfected into Expi293 cells via polyethyleneimine (PEI, 23966, Polysciences, Warrington, PA, USA) and cultured for 4–6 days after transfection. IgG-based proteins were purified with a protein A affinity chromatography (28408255, Cytiva, Marlborough, MA, USA) as previously described [30]. The HPN536 was purified using prepacked Ni2+ NTA columns (17524801, Cytiva) and eluted using 50 mM imidazole. The purification method of REGN7075 follows the description in previous literature [31]. Heterodimerization was conducted using Fab Arm Exchange (FAE) according to standard benchtop-scale procedures [32]. After Protein A affinity chromatography purification, the “2 + 1” structure BsAb was subjected to MMC ImpRes (17371620, Cytiva) mixed-mode chromatography to eliminate aggregates and byproducts. The anti-PD-L1 (Atezolizumab) mAb was generated and purified using the same procedure as described above.
Cell binding measurements
The binding of antibodies to cell surface-expressed antigens was determined by flow cytometry. In brief, a gradient dilution was performed for each antibody followed by a 40-min incubation at 4 °C with target cells. Subsequently, detection was carried out using PE anti-human IgG Fc (366904, Biolegend, San Diego, CA, USA) as the secondary antibody. Samples were acquired using a FACSCalibur (Beckman Coulter, Brea, CA, USA) and analyzed by GraphPad Prism 8.
In vitro T cell-dependent cell cytotoxicity
T cell-dependent cell cytotoxicity assays were conducted following previously described methods [33]. In brief, luciferase-expressing target cells were generated through lentiviral transduction, while effector cells were derived from human PBMCs. Under standard conditions, effector cells and target cells were co-seeded in a flat-bottom 96-well plate at a 10:1 ratio. Based on our research, the combination of TCB and TAA × CD28 BsAbs shows the most significant difference between the combination therapy and monotherapy groups at a 2:1 effector-to-target ratio (Supplementary Fig. S9). Therefore, we will maintain this ratio in future experiments. Target cell viability was evaluated using the Steady Glo Reagent (E2520, Promega, Madison, WI, USA). For the HT29-MSLN cells, the effector-to-target cell ratio was set at 10:1, followed by incubation at 37 °C for 40 h. Subsequently, PBMCs were carefully washed away, and viability was measured using the Cell Titer Glo luminescent cell viability reagent (G7570, Promega). The resulting data were analyzed using GraphPad Prism 8.
T cell activation and cytokine release determination
OVCAR3 or NCI-N87 cells, along with PBMCs, were seeded into individual wells of a 96-well plate at an effector-to-target cell ratio of 10:1 and co-incubated for 20 h. Subsequently, effector cells and supernatant were collected. Effector cells were isolated through centrifugation and stained with the following anti-human antibodies: CD4 (555347, BD Biosciences, San Jose, CA, USA), CD8 (555366, BD Biosciences), and CD69 (555533, BD Biosciences) for 30 min at 4 °C. After staining, cells were washed with PBS and then analyzed using a FACSCalibur flow cytometer. Cytokines concentrations in the supernatants were determined using an IFNγ (DY285B-05) and IL-2 (DY202-05) immunoassay kits from R&D Systems (Minneapolis, MN, USA), according to the manufacturer’s protocol.
Jurkat-NFAT luciferase reporter cell assay
After Jurkat-NFAT luciferase receptor cells (J1601, Promega) were cocultured with tumor cells at a ratio of 10:1 (Effector: target) for 16 h and treated with progressively diluted TCBs, subsequently, 100 μL of Steady Glo Reagent was introduced into each well and the plates were incubated for 10 min. Luminescence signals were quantified using the Tecan Plate Readers (TECAN, Männedorf, Switzerland) and the resulting data were analyzed using GraphPad Prism 8.
Antigen expression levels on tumor cells
We utilized flow cytometry to investigate the surface expression of relevant markers on tumor cell lines. For the detection of MSLN, we employed LP2/HP4-44 as the primary antibody, while REGN7075 and TF × CD28 served as primary antibodies for EGFR and TF, respectively. Subsequently, PE-conjugated anti-human IgG Fc was used as the secondary antibody for detection. For PD-L1 staining on NCI-N87 cells, the cells were collected following a 20-h incubation period with or without MSLN490 in the presence or absence of PBMCs. The stained cells were labeled using an APC-conjugated anti-PD-L1 antibody (10084-MM36-A, Sino Biological, Beijing, China) and data acquisition and analysis were conducted using a FACSCalibur.
Mice
All research procedures received approval from the Institutional Animal Care and Use Committee (IACUC) at the School of Pharmacy, Shanghai Jiao Tong University (Shanghai, China) and were conducted in strict accordance with the relevant animal welfare and protection standards. Male BALB/c mice and female NOD/SCID and NOG mice were procured from Charles River Laboratories (Beijing, China) and were aged between 6 and 8 weeks at the commencement of the experiments. The mice were housed under specific pathogen-free conditions with a 12-h light and 12-h darkness cycle. Upon their arrival, the animals underwent a 1-week acclimatization period to adapt to their new environment. Continuous daily health monitoring was conducted throughout the study.
Pharmacokinetics analysis
For pharmacokinetic analysis, single-dose pharmacokinetic analysis was performed in BALB/c male mice at a dose of 5 mg/kg for both MSLN490 and MSLN530 via intravenous injection. Blood serum was collected at the following time points: 0 (predose), 30 min, 6 h, 1, 3, 5, 7, 10, 15, 21, and 28 days. Blood concentrations of TCBs were determined using enzyme-linked immunosorbent assay (ELISA). Pharmacokinetic parameters were calculated using the PK Solver.
Tumor/PBMC co-grafting setting
NCI-N87 cells (5 × 106 cells) were mixed with PBMCs at the specified effector: target (E:T) ratios of 1:3 or 1:4. The tumor cells/PBMC mixture was subcutaneously implanted into female NOD/SCID mice (s.c.) in a total volume of 100 μL of RPMI-1640 medium. NOD/SCID mice were randomized based on body weight. MSLN490 and MSLN530 (0.2, 0.02, and 0.002 mg/kg, i.v.) were administered via weekly for 3 weeks, while HPN536 (0.2 mg/kg, i.v.) was administered daily for ten consecutive days. In the combination study with TAA × CD28, TAA × CD28 was dosed at 3 mg/kg (i.v.) once a week for 3 weeks, and MSLN490 was dosed at 0.03 mg/kg (i.v.) once a week for 3 weeks. The same dosing methods were applied in the combined MSLN490 with TAA × CD28 group. All groups received a consistent administration volume of 200 μL. Tumor volume (TV) was calculated according to the formula: TV (mm3) = (Length × Width2)/2.
Tumor model with intraperitoneal transfer of PBMC
Female NOG mice received subcutaneous implants of tumor cell lines into the right flank. After palpable tumors were established (100–150 mm3), randomization was performed based on both tumor volume and body weight. Subsequently, each mouse received an intravenous injection of 5 × 106 human PBMCs.
For the AsPC-1 xenograft model (2 × 106 cells per mouse), a 3-day interval followed the PBMC injection, after which MSLN490 was administered via tail vein injection once weekly for 3 weeks at doses of 3, 0.3, and 0.03 mg/kg, respectively. HPN536 was given at a daily dose of 0.8 mg/kg (i.v.) for 10 days.
In the case of the NCI-N87 xenograft model (5 × 106 cells per mouse), after a 3-day interval following PBMC injection, the Atezolizumab group received a dosage of 10 mg/kg (i.p.), while the MSLN490 group received a dosage of 0.2 mg/kg (i.v.). The mice in combination therapy group were administered with the same dosages. All treatment groups received treatment once weekly, totaling 3 weeks.
In the combination study with paclitaxel (S1150, Selleck Chemicals, Houston, TX, USA), following the establishment of the NCI-N87 xenograft model (5 × 106 cells per mouse), intravenous administration of paclitaxel was conducted at a dosage of 20 mg/kg (i.v.) on the day before PBMC injection. On the second day following PBMC injection, MSLN490 was intravenously administered at a dose of 1 mg/kg, once weekly, for a consecutive 3-week period.
All groups received a consistent administration volume of 200 μL. The tumor volume was estimated using the method described above.
Immunohistochemistry (IHC) analysis
Tumor samples obtained from euthanized animals were subjected to fixation in 4% PFA (G1101, Servicebio, Wuhan, China) for a duration of 24 h followed by paraffin embedding. Briefly, sections with a thickness of 4 micrometers were sliced using a Microtome (Leica Biosystems, Wetzlar, Germany) and affixed onto glass slides. The specimens underwent deparaffinization, and a heat-mediated antigen retrieval process was executed prior to immunostaining for human MSLN (ab196235, Abcam, Cambridge, UK), human CD3 (GB13440, Servicebio) and human CD8 (ab245118, Abcam). Hematoxylin (G1004, Servicebio) was employed for counterstaining, and slide scanning was carried out using the Pannoramic SCAN system (3DHISTECH, Budapest, Hungary).
Statistical analysis
Data were presented as mean ± standard derivation. Difference between two or multiple groups was compared by Student’s test or One-way ANOVA followed by Turkey’s test, respectively. P < 0.05 was considered statistically significant.
Results
MSLN affinity directly affects killing activity of IgG-Based TCBs in vitro
To generate antibodies with high affinity against MSLN, we utilized CDR walking and phage display methodologies to enhance the affinity from the parental antibody M912. We screened and identified four molecules from a pool with varying affinities towards MSLN. The corresponding affinity data for these anti-MSLN antibodies were presented in the Supplementary Table S1. The cell surface binding of the antibodies to MSLN was also confirmed by flow cytometry on HT-29-hMSLN cells, an engineered cell line with significant overexpression of exogenous MSLN (Fig. 1a). As indicated in Fig. 1b, the mAbs labeled HP4-44, HP1-A13, LP2/HP4-44 and MSLN-39 show significantly improved cellular-level binding to MSLN compared to M912. To investigate the impact of targeting MSLN affinity on the biological activity of IgG-Based TCBs, we generated the IgG heterodimer through controlled Fab-arm exchange [32]. As illustrated in Fig. 1c, d, among the TCBs tested, MSLN-39/CD3 exhibited the strongest binding ability to cell surface antigens, followed by LP2/HP4-44/CD3. Furthermore, results from the cell binding assay on Jurkat cells indicated that different types of TCBs exhibited similar binding to the CD3 on the cell surface. The identical outcomes had been corroborated in SPR experiments (Supplementary Table S2). To assess the efficacy of IgG-Based TCBs, we conducted in vitro cytotoxicity assays wherein the TCBs were expected to activate PBMCs to inhibit MSLN-expressing OVCAR3 ovarian cancer cell growth. Our results, as illustrated in Fig. 1e, demonstrated that increasing the affinity of the antibodies to MSLN significantly enhanced the cytotoxicity of IgG-Based TCB-mediated T cells against OVCAR3 cells. Specifically, MSLN-39/CD3 (EC50: 5.9 pM) and LP2/HP4-44/CD3 (EC50: 25.9 pM) exhibited more than 800-fold and 190-fold higher activity, respectively, compared to that of M912/CD3 (EC50: 5 nM). The activation of T cells by IgG-Based TCBs was investigated in a nuclear factor of activated Jurkat NFAT-Luc reporter bioassay (Fig. 1f). MSLN-39/CD3 and LP2/HP4-44/CD3 exhibited the most effective activation of CD3-mediated NFAT signaling in the presence of OVCAR3 cells. In summary, these findings demonstrate that MSLN-39/CD3 and LP2/HP4-44/CD3 with higher affinity to MSLN presented significant improved cytotoxicity compared to M912/CD3.
Fig. 1. Functional characterization of IgG-Based TCBs in vitro.
a Cell surface expression of MSLN determined using flow cytometry with HP4-44/LP2 antibody. b Anti-MSLN mAbs binding to HT-29-hMSLN cells analyzed by flow cytometry. c Structural characteristics of IgG-Based TCB. d Binding of IgG-Based TCBs to HT-29-hMSLN cells and Jurkat cells analyzed by flow cytometry (n = 2). e Luciferase-labeled OVCAR3 cells incubated with PBMC (E:T ratio 10:1) and treated with varying amounts of IgG-Based TCBs. OVCAR3 cell viability was measured after 40 h (n = 3). f Jurkat-NFAT luciferase reporter cells co-cultured with OVCAR3 cells, treated with serially diluted IgG-Based TCBs, and luciferase activity was measured 16 h later (n = 3). Data presented as mean ± SD.
Cytotoxicity of MSLN-targeting TCB is enhanced by IgG-[L]-scFv format
Next in our investigation of TCB activity, we designed two different structure formats, “2 + 2” (MSL490, IgG-[L]-scFv) and “2 + 1” (Fig. 2a). LP2/HP4-44 was used in all three TCBs as the MSLN binding arm. Experimental findings revealed that LP2/HP4-44 does not bind to murine-derived MSLN (Supplementary Fig. S1a). We determined the binding kinetics of different TCB formats to MSLN and huCD3ε by SPR (Supplementary Tables S1, S2) and evaluated their binding to antigen-expressing cells using flow cytometry. Our results demonstrated that MSLN490 exhibited significantly higher binding avidity to HT-29-hMSLN and Jurkat cells in comparison to “2 + 1” or “1 + 1” formats (Fig. 2b). Further, in vitro cytotoxicity and T cell activation assays were performed to assess the functional characteristics of each TCB format. The data showed that MSLN490 exhibited superior efficacy in T cell-mediated killing of OVCAR3 cells compared to “2 + 1” and “1 + 1” TCBs (Fig. 2c). While MSLN490 demonstrated a stronger capacity to activate T cells in NFAT-Luc reporter cells (Fig. 2d), no significant differences were observed between MSLN490 and “2 + 1” TCBs in terms of detecting the upregulation of CD69 on T cell surface following co-culture with PBMC and OVCAR3 cells (Fig. 2e). Interestingly, the cytokine release experiment revealed that MSLN490 induced lower levels of IFN-γ and IL-2 release compared to the “2 + 1” format (Fig. 2f). Although there were no significant differences cytotoxicity towards NCI-N87 cells mediated by MSLN490 and “2 + 1” (Supplementary Fig. S2b), similar to the results on OVCAR3 cells, we found that cytokine release was lower mediated by MSLN490 than “2 + 1” format (Supplementary Fig. S2d). Moreover, the T cell activation was compared between the LP2/HP4-44-[H]-CD3 (“2 + 1” format) and MSLN490 (Supplementary Fig. S3a). Both LP2/HP4-44-[H]-CD3 and MSLN490 exhibited similar potency in activating Jurkat reporter cells when co-cultured with OVCAR3 cells (Supplementary Fig. S3b), However, MSLN490 demonstrated slightly greater potency when co-cultured with Capan-2 cells (EC50: 5.58 ng/mL vs EC50: 10.29 ng/mL in Supplementary Fig. S3c). In the cytotoxicity assay, MSLN490 displayed 30–40 fold greater potency than HP4-44 [H]-hOKT3 (Supplementary Fig. S3d). Additionally, a cytotoxicity was not observed in the Raji tumor cell line (MSLN-negative), underscoring the specificity of MSLN490 for tumor cells expressing MSLN (Fig. 2g). MSLN490 was shown to simultaneously bind to hMSLN and CD3 (Fig. 2h). These findings suggest that MSLN490 has the potential to improve T cell-mediated tumor cell killing with reducing cytokine release.
Fig. 2. Functional characterization of different formats of TCB in vitro.
a Structural characteristics of TCBs. b Binding of TCBs to HT-29-hMSLN cells and Jurkat cells analyzed by flow cytometry (n = 2). c Luciferase-labeled OVCAR3 cells incubated with PBMC (E:T ratio 10:1) and treated with varying amounts of TCBs. OVCAR3 cell viability was measured after 40 h (n = 3). d Jurkat-NFAT luciferase reporter cells co-cultured with OVCAR3 cells, treated with serially diluted IgG-based TCBs, and luciferase activity was measured 16 h later. OVCAR3 cells co-cultured with PBMC (E:T ratio 10:1) were incubated with various concentrations of TCBs for 20 h (n = 3). e Percentages of CD69+/CD4+ and CD69+/CD8+ were determined by flow cytometry (n = 2). f IFNγ and IL-2 levels were also measured in the conditioned media collected from the cocultures by ELISA (n = 2). g Luciferase-labeled NCI-N87 and Raji cells incubated with PBMC (E:T ratio 10:1) and treated with varying amounts of TCBs. Cell viability was measured after 40 h (n = 3). h Simultaneous binding by surface plasmon resonance to both hMSLN and CD3. Data presented as mean ± SD.
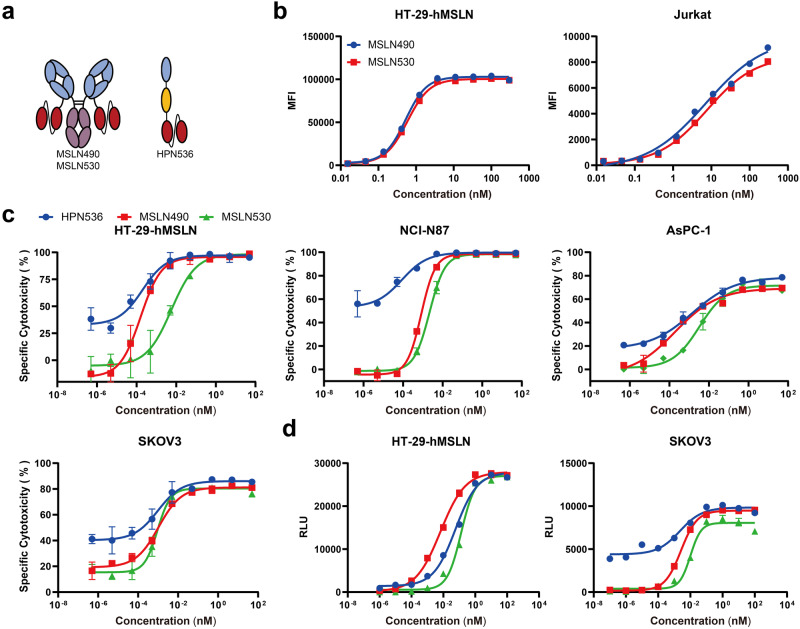
Higher MSLN affinity in MSLN530 does not translate to superior antitumor activity compared to MSLN490 in vitro
When comparing IgG-based TCBs, MSLN39/CD3-mediated T cells showed higher cytotoxicity than those mediated by LP2/HP4-44/CD3 (Fig. 1e). Consequently, we developed the MSLN530, incorporating a targeting domain derived from MSLN-39 (Fig. 3a), to investigate whether MSLN530 demonstrates superior cytotoxicity compared to MSLN490. Additionally, we employed HPN536 [16] as a positive control. Despite detecting a higher affinity of MSLN530 towards MSLN compared to MSLN490 by SPR (Supplementary Table S1), flow cytometric evaluation revealed no significant disparity in their binding affinity to cell-surface MSLN and CD3 (Fig. 3b). However, a moderate enhancement in the plateau phase was observed for MSLN530 relative to the MSLN490 specifically on NCI-N87 and SKOV3 cell lines (Supplementary Fig. S4a). Additionally, we constructed M912 (IgG-[L]-scFv) and HP1-A13 (IgG-[L]-scFv) based on M912 and HP1-A13 mAbs, respectively. As shown in Supplementary Fig. S5a, b, compared to M912 (IgG-[L]-scFv) and HP1-A13 (IgG-[L]-scFv), MSLN490 and MSLN530 significantly enhanced the cytotoxicity mediated by T cells against Capan-2 cells. However, we also observed no significant differences between MSLN490 and MSLN530 (Fig. 3c and Supplementary Fig. S6b) in cytotoxicity assays targeting tumor cells expressing endogenous MSLN. Nonetheless, in HT-29-hMSLN cells, MSLN490 (EC50: 0.16 pM,) exhibited a 35-fold higher antitumor cytotoxicity compared to MSLN530 (EC50: 5.707 pM). These results indicated that excessively enhancing the affinity for MSLN of IgG-[L]-scFv did not augment its cytotoxicity in vitro. T cell activation was assessed by using an NFAT reporter assay (Fig. 3d and Supplementary Fig. S6c), demonstrating comparable activation of T cell receptor (TCR) luciferase signals by MSLN490 and MSLN530. Consistently, similar observations were found in the activation of both CD4+ and CD8+ T cells (Supplementary Fig. S6d). T-cell activation was also evaluated by measuring the secretion of IFN-γ and IL-2, revealing no significant differences between MSLN490 and MSLN530 (Supplementary Fig. S6e). Additionally, we compared the two TCBs on cytotoxicity and T-cell activation with HPN536 and found that HPN536 exhibited superior activity in vitro.
Fig. 3. Higher MSLN affinity in MSLN530 does not translate to superior antitumor activity compared to MSLN490 in vitro.
a Structural characteristics of TCBs. b Binding of MSLN490 and MSLN 530 to HT-29-hMSLN cells and Jurkat cells analyzed by flow cytometry (n = 2). c Luciferase-labeled tumor cells incubated with PBMC (E:T ratio 10:1) and treated with varying amounts of TCBs. Cell viability measured after 40 h (n = 3). d Jurkat-NFAT luciferase reporter cells co-cultured with tumor cells, treated with serially diluted TCBs, and luciferase activity was measured 16 h later (n = 3). Data presented as mean ± SD.
Efficacy of MSLN490 is resistant to sMSLN interference
Studies indicate that the extracellular portion of membrane-bound MSLN has the potential to be released from tumor cells. Notably, individuals with malignant mesothelioma exhibit elevated serum concentrations of MSLN, median level reaching up to 2.7 nM [34]. These sMSLN could potentially impede the effectiveness of targeted immunotherapies by functioning as antigen traps. To assess the influence of sMSLN, we exposed SKOV3 and NCI-N87 cell lines to different TCBs while gradually increasing the concentrations of sMSLN (Fig. 4a). As shown in Fig. 4b, c, the cytotoxicity of T cells mediated by HPN536 is significantly influenced by sMSLN. In SKOV3 and NCI-N87 cells, the activities were decreased by 6.8-fold and 28-fold (EC50) when 20 ng/mL of sMSLN was added into the assay system. At the concentration of sMSLN reaching 200 ng/mL, the activity was decreased by a remarkable 55.9-fold and 169.3-fold (EC50). Also, we observed that MSLN530 was more sensitive to sMSLN compared to MSLN490. In the presence of 200 ng/mL sMSLN in the culture supernatant, the killing activities against SKOV3 and NCI-N87 cells were decreased by 50-fold and 27-fold (EC50), respectively (Fig. 4b, c). However, even at high concentrations (200 ng/mL) of sMSLN, there was no significant reduction in MSLN490-mediated T cell-killing activity against target cells (Fig. 4b, c). Based on the results, we speculate that TCB with IgG-[L]-scFv, due to their dual antigen binding arms, may have advantages over HPN536 with a monomeric T cell engager. However, this antibody structure does not fully resist the interferences from exogenous antigens. When the affinity of antigen binding in IgG-[L]-scFv is excessively high, it can also be significantly affected by sMSLN.
Fig. 4. Comparison of cytotoxicity in the presence of sMSLN between the TCBs.
a Experimental procedure schematic diagram. Tumor cells and PBMCs were co-cultured for 40 h in the presence or absence of the indicated amounts of sMSLN, cell viability measured by measuring luciferase activity. Target cell: NCI-N87 (b). Target cell: SKOV3 (c). Data presented as mean ± SD, n = 3.
Pharmacokinetics of MSLN490 and MSLN530 in BALB/c mice
To assess druggability of the TCBs, single-dose pharmacokinetic analysis was performed in BALB/c male mice at a dose of 5 mg/kg via intravenous injection. ELISA was employed to detect the plasma concentrations of MSLN490 and MSLN530 (Fig. 5a). MSLN490 exhibited high exposure, as evidenced by a greater area under the curve (AUC) and lower clearance (CL) values compared with MSLN530. The half-life of MSLN490 was ~8.68 days, while MSLN530 had a half-life of 6.58 days, indicating excellent stability of MSLN490 in vivo compared to MSLN530 (Fig. 5b).
Fig. 5. MSLN490 exhibits stronger anti-tumor effects in vivo.
a Schematic diagram of the enzyme-linked immunosorbent assay used to determine PK. b PK parameters of MSLN490 and MSLN530 in BALB/c mice. Data presented as mean ± SD, n = 5. c Schematic of treatment schedule and antitumor effects of TCBs in the NCI-N87/PBMC co-grafting model (n = 5). d Tumor volume and mice weight measured throughout the study, shown in mean (SEM). Statistical significance of tumor volumes at the end point was calculated using unpaired, two-tailed Student’s t test (*P < 0.05 and **P < 0.01). e Schematic of treatment schedule and antitumor effects of TCBs in the AsPC-1 xenograft model with intravenous transfer of PBMC (n = 6). f Tumor volume and mice weight measured throughout the study, shown in mean (SEM). Statistical significance of tumor volumes at the end point was calculated using unpaired one-way ANOVA with Tukey’s multiple comparison test (*P < 0.05, ***P < 0.001 and ****P < 0.0001). g Representative immunohistochemistry staining for human MSLN (left) and human CD3 (right) is shown. Tumors were collected at the end of the experiment described in (e). Scale bars are indicated in each panel.
MSLN490 exhibits stronger anti-tumor effects in vivo
The antitumor activities of MSLN490 and MSLN530 were assessed with the NCI-N87/PBMC co-grafting model in a prophylactic treatment setting (Fig. 5c). The BsAbs MSLN490 and MSLN530 were administered weekly, whereas HPN536 was given continuously due to its shorter half-life in mice [16]. As depicted in Fig. 5d, MSLN490 demonstrated significant tumor growth inhibition at a dose of 0.2 mg/kg. Notably, at the same 0.2 mg/kg dose, MSLN530 did not show significant tumor growth inhibition, although MSLN530 has higher affinity than that of MSLN490 for MSLN. When compared to the positive control HPN536, MSLN490 displayed superior anti-tumor efficacy. Next, further investigations of anti-tumor efficacy of MSLN490 in the AsPC-1 tumor model in a delayed or therapeutic treatment was conducted (Fig. 5e) and results have been illustrated in Fig. 5f, which displayed dose-dependent inhibition of tumor growth by MSLN490 and notably surpassed HPN536 at equimolar concentrations. Moreover, MSLN490 administration in these two xenograft models did not result in significant body weight loss, indicating low cytotoxicity in vivo.
To investigate the infiltration of lymphocytes within the tumor tissue, tumor samples were collected for immunohistochemical analysis, after the completion of the AsPC-1 tumor model experiment. Remarkably, at high dosage (3 mg/kg), MSLN490 significantly promoted lymphocyte infiltration within the tumor, thereby validating its potent anti-tumor efficacy in the AsPC-1 tumor model (Fig. 5g). Therefore, we conclude that MSLN490 exhibits stronger anti-tumor effects in vivo.
Combination of MSLN490 with Atezolizumab in vivo
Our research findings demonstrated that co-culturing NCI-N87 cells with PBMC in the presence of MSLN490 led to an upregulation of PD-L1 expression on NCI-N87 cells (Supplementary Fig. S7a), which led us to further investigate combo effectiveness with anti-PD-L1 mAb. In vitro experiments indicated that the addition of Atezolizumab moderately enhanced the cytotoxic activity of MSLN490 (Supplementary Fig. S7b). Because anti-tumor efficacy was observed with MSLN490 at 0.2 mg/kg for the prophylactic treatment of NCI-N87 tumors (Fig. 5d), we decided to examine combinatorial efficacy in the context of delayed or therapeutic treatment. Surprisingly, combo treatment of MSLN490 with Atezolizumab in the context of delayed or therapeutic treatment didn’t enhance the anti-tumor efficacy of MSLN490, which we never expected (Fig. 6a, b). It might be due to the dose used in the experiment was too high (Atezolizumab at 10 mg/kg and MSLN490 at 0.2 mg/kg) to distinguish the differences in this tumor model. Immunohistochemical analysis of the tumors harvested after completion of the study revealed a crucial difference that emerged at the end of the study. As shown in Fig. 6c and Supplementary Fig. S8a, in the combination group, all MSLN-positive tumor cells were completely eradicated within the tumors, while still some remained in the MSLN490 alone group. Additionally, we also observed increased infiltration of T cells in the combination group. Thus, in combination with Atezolizumab, MSLN490 demonstrated enhanced anti-tumor effects, offering a promising strategy to enhance MSLN-490’s anti-tumor activity in future research.
Fig. 6. Combination of MSLN490 with Atezolizumab or TAA×CD28 BsAbs in vivo.
a Schematic of treatment schedule and antitumor effects of TCBs in the NCI-N87 xenograft model (n = 8). b Tumor volume and mice weight measured throughout the study, shown in mean (SEM). Statistical significance of tumor volumes at the end point was calculated using unpaired one-way ANOVA with Tukey’s multiple comparison test (*P < 0.05, ***P < 0.001, and ****P < 0.0001). c Representative immunohistochemistry staining for human MSLN (left), human CD3 (middle) and human CD8 (right) is shown. Tumors were collected at the end of the experiment. Scale bars are indicated in each panel. d Flow cytometry analysis of EGFR and TF cell surface expression on NCI-N87 cells. e Luciferase-labeled NCI-N87 cells were incubated with PBMC (E:T ratio 2:1) with a dose titration of MSLN490 and a fixed concentration of TAA × CD28 (2.5 μg/mL). Cell viability was measured by measuring luciferase activity. Data presented as mean ± SD, n = 3. f Schematic of treatment schedule in the NCI-N87 xenograft model (n = 6). g Tumor volume and mice weight measured throughout the study, shown in mean (SEM). Statistical significance of tumor volumes at the end point was calculated using unpaired one-way ANOVA with Tukey’s multiple comparison test (*P < 0.05, **P < 0.01).
TAA × CD28 BsAbs enhance the antitumor activity of the MSLN490 in NCI-N87 xenograft model
In order to activate T cells effectively, two signals from the TCR complex and from co-stimulatory receptors may be required [35]. While MSLN490 provides Signal 1 for T cell activation, additional co-stimulatory signals, such as a CD28 agonist may enhance T cell activation. Research suggests that combining TCB with TAA × CD28 BsAbs improves anti-tumor effects [36, 37]. To investigate if MSLN490 combined with TAA × CD28 BsAbs enhanced anti-tumor activity, we evaluated the tumor inhibitory effect on NCI-N87 by combining MSLN490 with BsAbs REGN7075 (EGFR × CD28) described previously or TF × CD28 [38]. In Fig. 6d, it was evident that NCI-N87 cells expressed EGFR and TF on their surface. As depicted in Fig. 6e, both REGN7075 and TF × CD28 enhanced the anti-tumor efficacy of MSLN490 in vitro significantly. In order to investigate the combinatorial efficacy, we selected a low dose of MSLN490 (0.03 mg/kg) for prophylactic treatment in NCI-N87 tumors. Interestingly, none of the BsAb alone, REGN7075 (3 mg/kg) or TF × CD28 (3 mg/kg), or MSLN490 showed significant anti-tumor activity, while, a significant anti-tumor activity was observed with both combo groups (Fig. 6f, g).
Combination of MSLN490 with paclitaxel increases anti-tumor efficacy in noninflamed tumors
TCB therapy depends on the accessibility of immune cells to tumor tissue. Nonetheless, in “cold tumors” characterized by insufficient immune cell infiltration, this treatment is ineffective. Increasing evidence suggests that combining immunotherapy with chemotherapy can ameliorate this situation [39]. Therefore, we further investigated tumor-inhibitory effect by combination of MSLN490 and paclitaxel, a second-line treatment for gastric carcinoma. There was a recent report that during TCB therapy, the MKN45 tumor model displays reduced immune cells infiltration compared to other tumor models, potentially impacting TCB efficacy [40]. As shown in Fig. 7a, MKN45 cells exhibited low MSLN expression. In vitro cytotoxicity experiments confirmed that MSLN490 effectively mediated T cell cytotoxicity against MKN45 (Fig. 7b).
Fig. 7. Paclitaxel enhances the antitumor activity of the MSLN490 in MKN45 xenograft model.
a Flow cytometry analysis of MSLN cell surface expression on MKN45 cells. b Luciferase-labeled tumor cells incubated with PBMC (E:T ratio 10:1) and treated with varying amounts of MSLN490. Cell viability measured after 40 h (n = 3). Data presented as mean ± SD. c Schematic of treatment schedule in the MKN45 xenograft model (n = 6). d Tumor volume measured throughout the study, shown in mean (SEM). e Tumor volume of individual mice in each group measured throughout the study. f Digital image of the stripped tumors. Statistical significance of tumor volumes at the end point was calculated using unpaired one-way ANOVA with Tukey’s multiple comparison test (*P < 0.05, **P < 0.01). g Representative immunohistochemistry staining for human MSLN (left) and human CD3 (right) is shown. Tumors were collected at the end of the experiment. Scale bars are indicated in each panel.
Inspiringly, a synergistic effect of the combo treatment in the MKN45 tumor model was observed (Fig. 7c–f), while either MSLN490 (1 mg/kg) or paclitaxel (20 mg/kg) did not exhibit a significant anti-tumor effect compared with the negative control of PBS buffer. To understand the mechanism of action, tumor immunohistochemistry was performed (Fig. 7g). Minimal MSLN staining in all four groups confirmed low MSLN expression in MKN45 tumor cells. CD3 staining result indicated minimal T cell infiltration in tumors from the PBS, paclitaxel, and MSLN-490 groups, suggesting that MKN450 is an immune-desert tumor. Therefore, treatment with MSLN-490 or paclitaxel did not alter this characteristic. In contrast, the combination therapy group exhibited a substantial infiltration of T cells into tumors, consistent with the inhibition of tumor growth, indicating a synergistic effect of the combination treatment. The synergistic effect could result from paclitaxel-induced killing of outer layer tumor cells, facilitating enhanced infiltration of immune cells by MSLN490.
Discussion
TCBs have emerged as promising cancer therapeutics, with numerous reports showing promising clinical potential for this strategy [41–45]. We generated a pool of mAbs specifically interacting with MSLN by CDR walking and phage display techniques derived from the parental antibody M912. Subsequently, we evaluated several different formats of the TCBs, including “1 + 1”, “2 + 2”, and “2 + 1”. TCBs with the IgG-[L]-scFv structure have been reported to enhance TCB efficacy significantly without inducing non-specific activation of T cells [25, 26]. Previously, we have demonstrated the efficacy of the IgG-[L]-scFv framework in eliciting anti-tumor responses [46]. Results from this report confirmed that, when compared to “1 + 1” TCBs, MSLN490 significantly boosted T cell cytotoxicity against tumor cells. Related research has shown that “2 + 1” TCB structures also demonstrate remarkable anti-tumor effects and, due to spatial hindrance, may reduce the CD3 end affinity, potentially lower cytokine storm and other side effects [47]. We conducted in vitro comparisons between MSLN490 and “2 + 1” TCB, the results showed that MSLN490 had higher tumor cell cytotoxicity with reduced cytokine release. This provides additional evidence for the efficacy and safety of IgG-[L]-scFv structure TCBs.
We also investigated the impact of affinity targeting MSLN on the biological activity of lgG-[L]-scFv structure TCBs. Despite MSLN490 and MSLN530 having different affinities for MSLN, with values of 12.3 nM and 3.55 nM, respectively, there were no significant differences in their cell binding levels. This is likely attributed to the avidity-mediated binding of these TCBs to the cell surface MSLN. Furthermore, both TCBs exhibited quite comparable cytotoxicity against tumor cells.
MSLN is a GPI-linked cell-surface glycoprotein highly expressed in many human cancers [12, 48]. The concentration of sMSLN in the blood of MSLN-positive cancer patients increases [18, 34]. This is mainly attributed to the ease with which MSLN is enzymatically cleaved and released into the bloodstream. Excitingly, our in vitro research revealed that high concentrations of sMSLN had an insignificant impact on the activity of MSLN490, either on SKOV3 or NCI-N87 cells, while sMSLN inhibited the cytotoxic activity of T cells mediated by HPN536 and MSLN530. We speculate that the results may be attributed to the high affinity of the TCBs to MSLN. According to previous reports, HPN536 has an affinity of 0.21 nM to MSLN [16]. Additionally, the bivalency of MSLN490 in binding to MSLN further reduced the interference by sMSLN. Nevertheless, in the NCI-N87 mouse tumor in vivo model, we observed clearly that the anti-tumor activity of MSLN490 was significantly superior to that of MSLN530 and HPN536.
TCB-induced T cell activation has been shown to upregulate PD-1 expression on T cells and induce PD-L1 expression on tumor cells [46, 49]. Our results demonstrated that, although there was no significant difference between the combo treatment and MSLN490 monotherapy, immunohistochemical analysis revealed that the combo treatment essentially cleared the tumor cells, whereas still some remaining in the MSLN490 alone group. Additionally, we also observed increased infiltration of T cells in the combination group. Taken together, these results indicated that Atezolizumab enhanced the anti-tumor effects of MSLN490. CD28 is a co-stimulatory signal that can enhance TCBs activation and anti-tumor effects [36, 37, 50]. Our research demonstrated that combining MSLN490 with TAA × CD28 BsAbs, showing a significantly greater anti-tumor efficacy.
At present, although multiple TCBs have been approved for the market, effective treatment of solid tumors by TCBs is highly desirable. Many tumors, called “cold tumor” have no immune cell infiltration in the environment, causing complexity for immuno-treatment [51]. This presents a formidable challenge for TCBs targeting solid tumors. In this context, combining with chemotherapy holds promise [40, 52], as it can disrupt tumor structure, rendering it more receptive to TCB-mediated immunotherapy. Our findings confirmed that the co-administration of MSLN490 with paclitaxel not only significantly augmented anti-tumor efficacy but also remarkably enhanced immune cell infiltration. In summary, our findings have substantiated the robust anti-tumor efficacy of MSLN490. Furthermore, due to its appropriate affinity and the structural integrity of lgG-[L]-scFv, MSLN490 exhibits resilience against the influence of sMSLN. In combination with other therapeutic modalities, we evaluated the anti-tumor effects of MSLN490 when co-administered with Atezolizumab, TAA × CD28, and paclitaxel. The results demonstrated that combining MSLN490 with a variety of anti-tumor agents enhanced its anti-tumor potential, providing a practical basis for its future clinical applications.
Supplementary information
Acknowledgements
We would like to express our gratitude to Jecho Biopharmaceuticals and Jecho Laboratories, Inc. for their valuable technical support. This work was supported by the National Natural Science Foundation of China (Grant No. 81773621 and 82073751 to JWZ), and the National Science and Technology Major Project “Key New Drug Creation and Manufacturing Program” of China (Grant No. 2019ZX09732001-019 to JWZ).
Author contributions
JWZ, ZDP, YQX, SSW, HJ, and JJL conceptualized and designed the study. JJL and HYY conducted protein production. JJL, YLY, and JC performed in vitro assays. JJL, YLY, LW, and JYL carried out animal experiments. JJL, ZDP, and JWZ analyzed the experimental results. JJL, JWZ, JG, BHZ, MYW, YSY, and YLB contributed to writing, original drafts, review, and editing. All authors critically reviewed the manuscript.
Competing interests
MSLN490 is in drug development in Jecho Laboratories Inc. (MD, USA) and Jecho Biopharmaceuticals Co., Ltd (Tianjin, China). SSW, HJ, and YQX are employees of Jecho Laboratories Inc. JG is an employee of Jecho Biopharmaceuticals Co., Ltd. ZDP, JC, and HYY are employees of Jecho Institute (Shanghai, China). No potential conflicts of interest were disclosed by the other authors.
Footnotes
These authors contributed equally: Jun-jun Liu, Zhi-di Pan
Contributor Information
Yue-qing Xie, Email: yueqingxie@jecholabs.com.
Jian-wei Zhu, Email: jianweiz@sjtu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41401-024-01316-6.
References
- 1.Lugtenburg P, Mous R, Clausen MR, Chamuleau ME, Johnson P, Linton K, et al. First-in-human, phase 1/2 trial to assess the safety and clinical activity of subcutaneous GEN3013 (DuoBody®-CD3 × CD20) in B-cell non-Hodgkin lymphomas. Blood. 2019;134:758. [Google Scholar]
- 2.Lim EA, Schweizer MT, Chi KN, Aggarwal RR, Agarwal N, Gulley JL, et al. Safety and preliminary clinical activity of JNJ-63898081 (JNJ-081), a PSMA and CD3 bispecific antibody, for the treatment of metastatic castrate-resistant prostate cancer (mCRPC). J Clin Oncol. 2022;40:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bannerji R, Arnason JE, Advani RH, Brown JR, Allan JN, Ansell SM, et al. Odronextamab, a human CD20 × CD3 bispecific antibody in patients with CD20-positive B-cell malignancies (ELM-1): results from the relapsed or refractory non-Hodgkin lymphoma cohort in a single-arm, multicentre, phase 1 trial. Lancet Haematol. 2022;9:e327–e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weidanz J. Targeting cancer with bispecific antibodies. Science. 2021;371:996–7. [DOI] [PubMed] [Google Scholar]
- 5.Kang C. Mosunetuzumab: first approval. Drugs. 2022;82:1229–34. [DOI] [PubMed] [Google Scholar]
- 6.Frampton JE. Epcoritamab: First Approval. Drugs. 2023;83:1331–40. [DOI] [PubMed] [Google Scholar]
- 7.Keam SJ. Talquetamab: first approval. Drugs. 2023;83:1439–45. [DOI] [PubMed] [Google Scholar]
- 8.Singh A, Dees S, Grewal I. Overcoming the challenges associated with CD3+ T-cell redirection in cancer. Br J Cancer. 2021;124:1037–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Middelburg J, Kemper K, Engelberts P, Labrijn A, Schuurman J, van Hall T. Overcoming challenges for CD3-bispecific antibody therapy in solid tumors. Cancers. 2021;13:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herbst RS, Soria J-C, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gajewski TF, Woo S-R, Zha Y, Spaapen R, Zheng Y, Corrales L, et al. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Curr Opin Immunol. 2013;25:268–76. [DOI] [PubMed] [Google Scholar]
- 12.Hassan R, Bera T, Pastan I. Mesothelin: a new target for immunotherapy. Clin Cancer Res. 2004;10:3937–42. [DOI] [PubMed] [Google Scholar]
- 13.Baba K, Ishigami S, Arigami T, Uenosono Y, Okumura H, Matsumoto M, et al. Mesothelin expression correlates with prolonged patient survival in gastric cancer. J Surg Oncol. 2012;105:195–9. [DOI] [PubMed] [Google Scholar]
- 14.Hassan R, Kreitman RJ, Pastan I, Willingham MC. Localization of mesothelin in epithelial ovarian cancer. Appl Immunohistochem Mol Morphol. 2005;13:243–7. [DOI] [PubMed] [Google Scholar]
- 15.Hassan R, Thomas A, Alewine C, Le D, Jaffee E, Pastan I. Mesothelin immunotherapy for cancer: ready for prime time? J Clin Oncol. 2016;34:4171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molloy M, Austin R, Lemon B, Aaron W, Ganti V, Jones A, et al. Preclinical characterization of HPN536, a trispecific, T-cell-activating protein construct for the treatment of mesothelin-expressing solid tumors. Clin Cancer Res. 2021;27:1452–62. [DOI] [PubMed] [Google Scholar]
- 17.Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci USA. 1996;93:136–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho M, Onda M, Wang Q-C, Hassan R, Pastan I, Lively MO. Mesothelin is shed from tumor cells. Cancer Epidemiol Biomark Prev. 2006;15:1751. [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Chan A, Tai C-H, Andresson T, Pastan I. Multiple proteases are involved in mesothelin shedding by cancer cells. Commun Biol. 2020;3:728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Chertov O, Zhang J, Hassan R, Pastan I. Cytotoxic activity of immunotoxin SS1P is modulated by TACE-dependent mesothelin shedding. Cancer Res. 2011;71:5915–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X, Onda M, Watson N, Hassan R, Ho M, Bera TK, et al. Highly active CAR T cells that bind to a juxtamembrane region of mesothelin and are not blocked by shed mesothelin. Proc Natl Acad Sci USA. 2022;119:e2202439119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin I, Rupert PB, Pilat K, Ruff RO, Friend DJ, Chan MK, et al. Novel mesothelin antibodies enable crystallography of the intact mesothelin ectodomain and engineering of potent, T cell-engaging bispecific therapeutics. Front Drug Discov. 2023;3:1216516. [Google Scholar]
- 23.Labrijn AF, Janmaat ML, Reichert JM, Parren PWHI. Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov. 2019;18:585–608. [DOI] [PubMed] [Google Scholar]
- 24.Slaga D, Ellerman D, Lombana TN, Vij R, Li J, Hristopoulos M, et al. Avidity-based binding to HER2 results in selective killing of HER2-overexpressing cells by anti-HER2/CD3. Sci Transl Med. 2018;10:eaat5775. [DOI] [PubMed] [Google Scholar]
- 25.Park JA, Santich BH, Xu H, Lum LG, Cheung NKV. Potent ex vivo armed T cells using recombinant bispecific antibodies for adoptive immunotherapy with reduced cytokine release. J Immunother Cancer. 2021;9:e002222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santich BH, Park JA, Tran H, Guo HF, Huse M, Cheung NKV. Interdomain spacing and spatial configuration drive the potency of IgG-[L]-scFv T cell bispecific antibodies. Sci Transl Med. 2020;12:eaax1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng Y, Xiao X, Zhu Z, Streaker E, Ho M, Pastan I, et al. A novel human monoclonal antibody that binds with high affinity to mesothelin-expressing cells and kills them by antibody-dependent cell-mediated cytotoxicity. Mol Cancer Ther. 2009;8:1113–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Golfier S, Kopitz C, Kahnert A, Heisler I, Schatz C, Stelte-Ludwig B, et al. Anetumab ravtansine: a novel mesothelin-targeting antibody-drug conjugate cures tumors with heterogeneous target expression favored by bystander effect. Mol Cancer Ther. 2014;13:1537–48. [DOI] [PubMed] [Google Scholar]
- 29.Breij EC, de Goeij BE, Verploegen S, Schuurhuis DH, Amirkhosravi A, Francis J, et al. An antibody-drug conjugate that targets tissue factor exhibits potent therapeutic activity against a broad range of solid tumors. Cancer Res. 2014;74:1214–26. [DOI] [PubMed] [Google Scholar]
- 30.Han L, Chen J, Ding K, Zong H, Xie Y, Jiang H, et al. Efficient generation of bispecific IgG antibodies by split intein mediated protein trans-splicing system. Sci Rep. 2017;7:8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith EJ, Olson K, Haber LJ, Varghese B, Duramad P, Tustian AD, et al. A novel, native-format bispecific antibody triggering T-cell killing of B-cells is robustly active in mouse tumor models and cynomolgus monkeys. Sci Rep. 2015;5:17943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Labrijn AF, Meesters JI, Priem P, de Jong RN, van den Bremer ET, van Kampen MD, et al. Controlled Fab-arm exchange for the generation of stable bispecific IgG1. Nat Protoc. 2014;9:2450–63. [DOI] [PubMed] [Google Scholar]
- 33.Nazarian A, Archibeque I, Nguyen Y, Wang P, Sinclair A, Powers D. Characterization of bispecific T-cell Engager (BiTE) antibodies with a high-capacity T-cell dependent cellular cytotoxicity (TDCC) assay. J Biomolecular Screen. 2015;20:519–27. [DOI] [PubMed] [Google Scholar]
- 34.Linch M, Gennatas S, Kazikin S, Iqbal J, Gunapala R, Priest K, et al. A serum mesothelin level is a prognostic indicator for patients with malignant mesothelioma in routine clinical practice. BMC Cancer. 2014;14:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bretscher PA. A two-step, two-signal model for the primary activation of precursor helper T cells. Proc Natl Acad Sci USA. 1999;96:185–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei J, Montalvo-Ortiz W, Yu L, Krasco A, Olson K, Rizvi S, et al. CD22-targeted CD28 bispecific antibody enhances antitumor efficacy of odronextamab in refractory diffuse large B cell lymphoma models. Sci Transl Med. 2022;14:eabn1082. [DOI] [PubMed] [Google Scholar]
- 37.Skokos D, Waite JC, Haber L, Crawford A, Hermann A, Ullman E, et al. A class of costimulatory CD28-bispecific antibodies that enhance the antitumor activity of CD3-bispecific antibodies. Sci Transl Med. 2020;12:eaaw7888. [DOI] [PubMed] [Google Scholar]
- 38.Segal NH, Pelster M, Girda E, Fong L, Olszanski AJ, Han H, et al. A phase 1/2 study of REGN7075 (EGFR x CD28 costimulatory bispecific antibody) in combination with cemiplimab (anti-PD-1) in patients with advanced solid tumors: trial-in-progress update. J Clin Oncol. 2023;41:TPS277–TPS. [Google Scholar]
- 39.Heinhuis KM, Ros W, Kok M, Steeghs N, Beijnen JH, Schellens JHM. Enhancing antitumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors. Ann Oncol. 2019;30:219–35. [DOI] [PubMed] [Google Scholar]
- 40.Sano Y, Azuma Y, Tsunenari T, Kayukawa Y, Shinozuka J, Fujii E, et al. Combination of T cell-redirecting bispecific antibody ERY974 and chemotherapy reciprocally enhances efficacy against non-inflamed tumours. Nat Commun. 2022;13:5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Y, Zong H, Han L, Xie Y, Jiang H, Gilly J, et al. A novel bispecific antibody targeting CD3 and prolactin receptor (PRLR) against PRLR-expression breast cancer. J Exp Clin Cancer Res. 2020;39:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen J, Pan Z, Han L, Zhou Y, Zong H, Wang L, et al. A novel bispecific antibody targeting CD3 and Lewis Y with potent therapeutic efficacy against gastric cancer. Biomedicines. 2021;9:1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun R, Zhou Y, Han L, Pan Z, Chen J, Zong H, et al. A rational designed novel bispecific antibody for the treatment of GBM. Biomedicines. 2021;9:640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang L, Qiao Y, Zong H, Han L, Ke Y, Pan Z, et al. IgG-like bispecific antibody CD3× EpCAM generated by split intein against colorectal cancer. Front Pharmacol. 2022;13:803059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen J, Pan Z, Han L, Liu J, Yue Y, Xiao X, et al. Binding domain on CD22 molecules contributing to the biological activity of T cell-engaging bispecific antibodies. Heliyon. 2023;9:e17960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pan Z, Chen J, Xiao X, Xie Y, Jiang H, Zhang B, et al. Characterization of a novel bispecific antibody targeting tissue factor-positive tumors with T cell engagement. Acta Pharm Sin B. 2022;12:1928–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rius Ruiz I, Vicario R, Morancho B, Morales C, Arenas E, Herter S, et al. p95HER2-T cell bispecific antibody for breast cancer treatment. Sci Transl Med. 2018;10:eaat1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pastan I, Hassan R. Discovery of mesothelin and exploiting it as a target for immunotherapy. Cancer Res. 2014;74:2907–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sam J, Colombetti S, Fauti T, Roller A, Biehl M, Fahrni L, et al. Combination of T-cell bispecific antibodies with PD-L1 checkpoint inhibition elicits superior anti-tumor activity. Front Oncol. 2020;10:575737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Warwas KM, Meyer M, Gonçalves M, Moldenhauer G, Bulbuc N, Knabe S, et al. Co-Stimulatory bispecific antibodies induce enhanced T cell activation and tumor cell killing in breast cancer models. Front Immunol. 2021;12:719116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. [DOI] [PubMed] [Google Scholar]
- 52.Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18:197–218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.