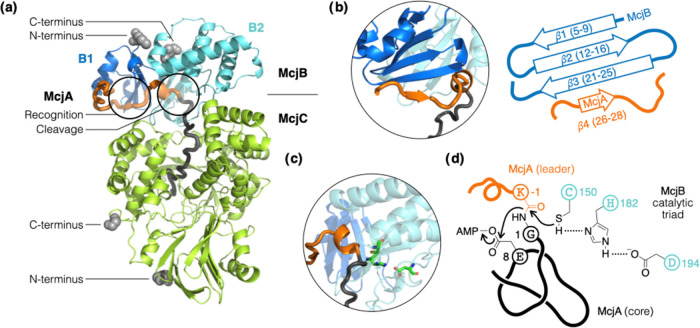
Figure 2.
AF2 predicts the formation of a McjA/McjB/McjC ternary complex. (a) The leader peptide interacts mostly with McjB and the core peptide extends into a deep cavity in McjC. The first 13 residues of McjB are omitted for clarity. The N- and C-termini of McjB and McjC are shown as gray spheres; the rest of the structures are shown in cartoon and color-coded the same way as in Figure 1. (b) Key to leader peptide recognition is an antiparallel β-sheet in the B1 domain of McjB. A short segment of the leader peptide (McjA(−12)to(−10)) aligns along β3 as a fourth strand to extend the β-sheet. (c) The B2 domain of McjB is a protease whose Cys-His-Asp catalytic triad (shown in sticks) is poised to cleave the amide bond that connects the leader and the core peptides. (d) The speculative mechanism of MccJ25 maturation is shown, wherein McjB catalyzes leader cleavage, and McjC catalyzes isopeptide bond formation to entrap the tail of the core peptide. All protein structures were rendered by PyMOL.