Figure 4.
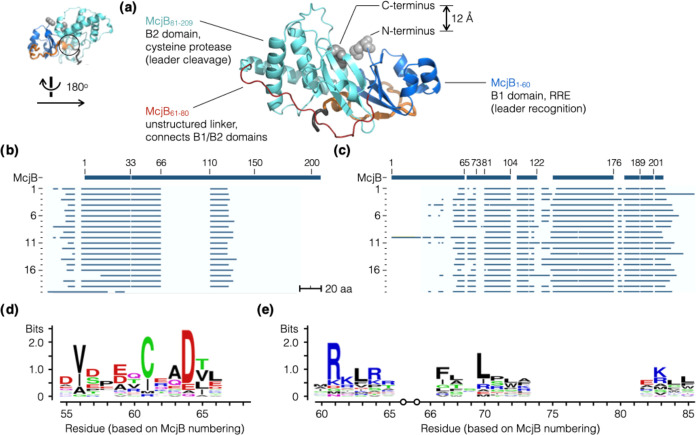
Identifying the B1/B2 domain boundary in McjB. (a) AF2 predicted McjB to fold into discrete domains, B1 (RRE, residues 1 to 60, blue) and B2 (protease, residues 81 to 208, cyan), that are connected by a linker with no apparent secondary structure (residues 61 to 80, red). Its N- and C-termini are located in close proximity and prompted us to test a series of circularly permutated and split variants of McjB. (b, c) We compiled a collection of phylogenetically diverse lasso peptide BGCs (20) that contain separate B1 and B2 proteins. The sequences of these B1 (b) and B2 proteins (c) were aligned to McjB; logo plots were generated for sequence alignments of B1 (d) and B2 (e). These results corroborated the predicted AF2 structure, suggesting that the domain boundary is likely somewhere in the 61–80 region.