Fig. 2 |. Scope of pyrimidinium salts, 2-substituted pyrimidines and 1,2-azoles.
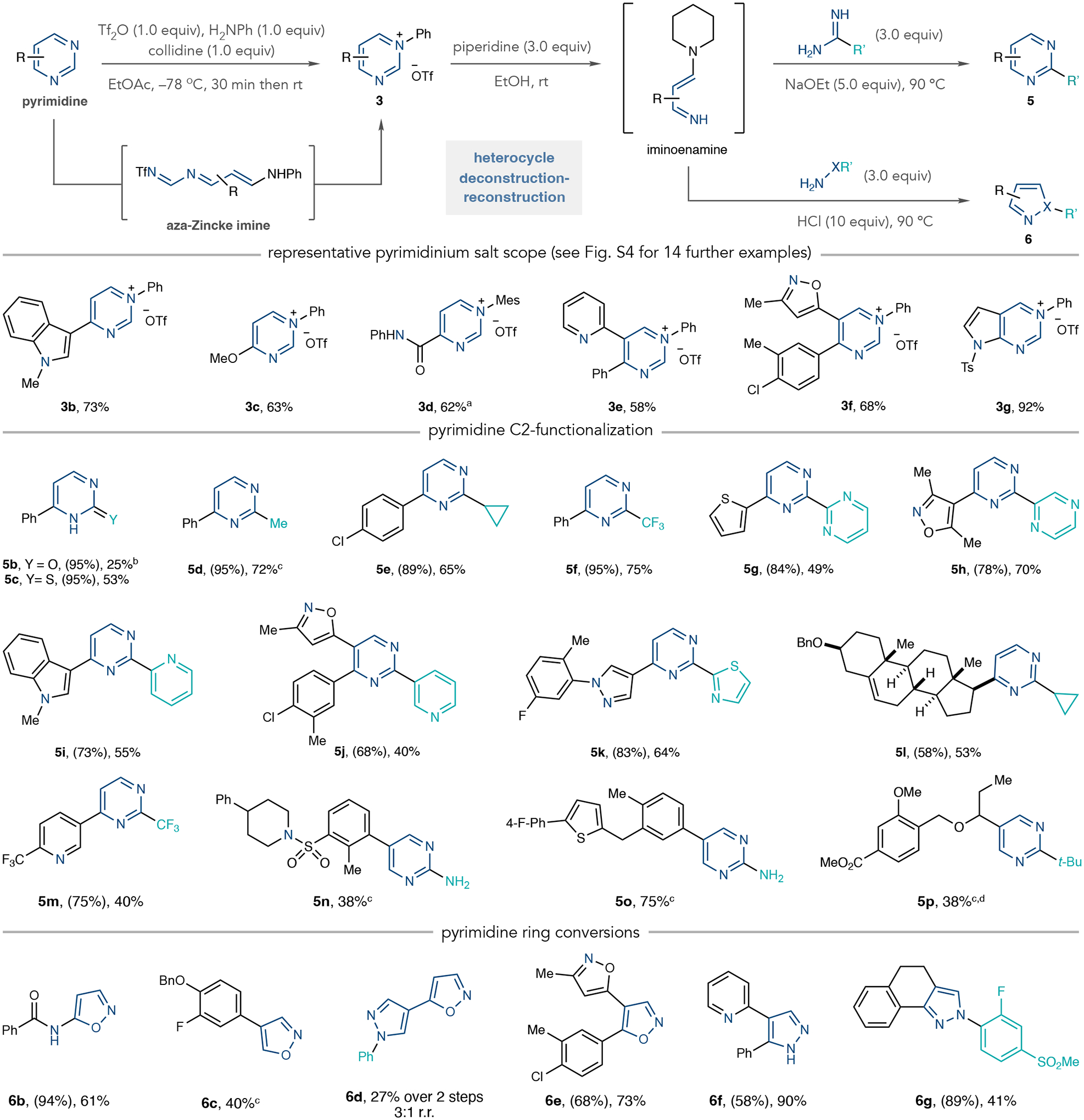
Isolated yields are shown. Yields in parentheses are for pyrimidinium salts. aInstead of aniline, 2,4,6-trimethylaniline was used. bInstead of piperidine, 6.0 equivalents of pyrrolidine was used. cIsolated as a 17:1 ratio with 1a. cOne-pot protocol: 4-nitroaniline used instead of aniline, then the solvent was exchanged for ethanol (EtOH). In the ring-closing step, 10–20 equivalents of amidine or hydroxylamine were used. dSodium methoxide (NaOMe) and methanol (MeOH) used instead of sodium ethoxide (NaOEt) and EtOH. Mes, 2,4,6-trimethylphenyl; Ts, tosyl; Bn, benzyl; t-Bu, tert-butyl.
