Fig. 3 |. Applications of the pyrimidine diversification strategy to biologically active molecules and further reaction development.
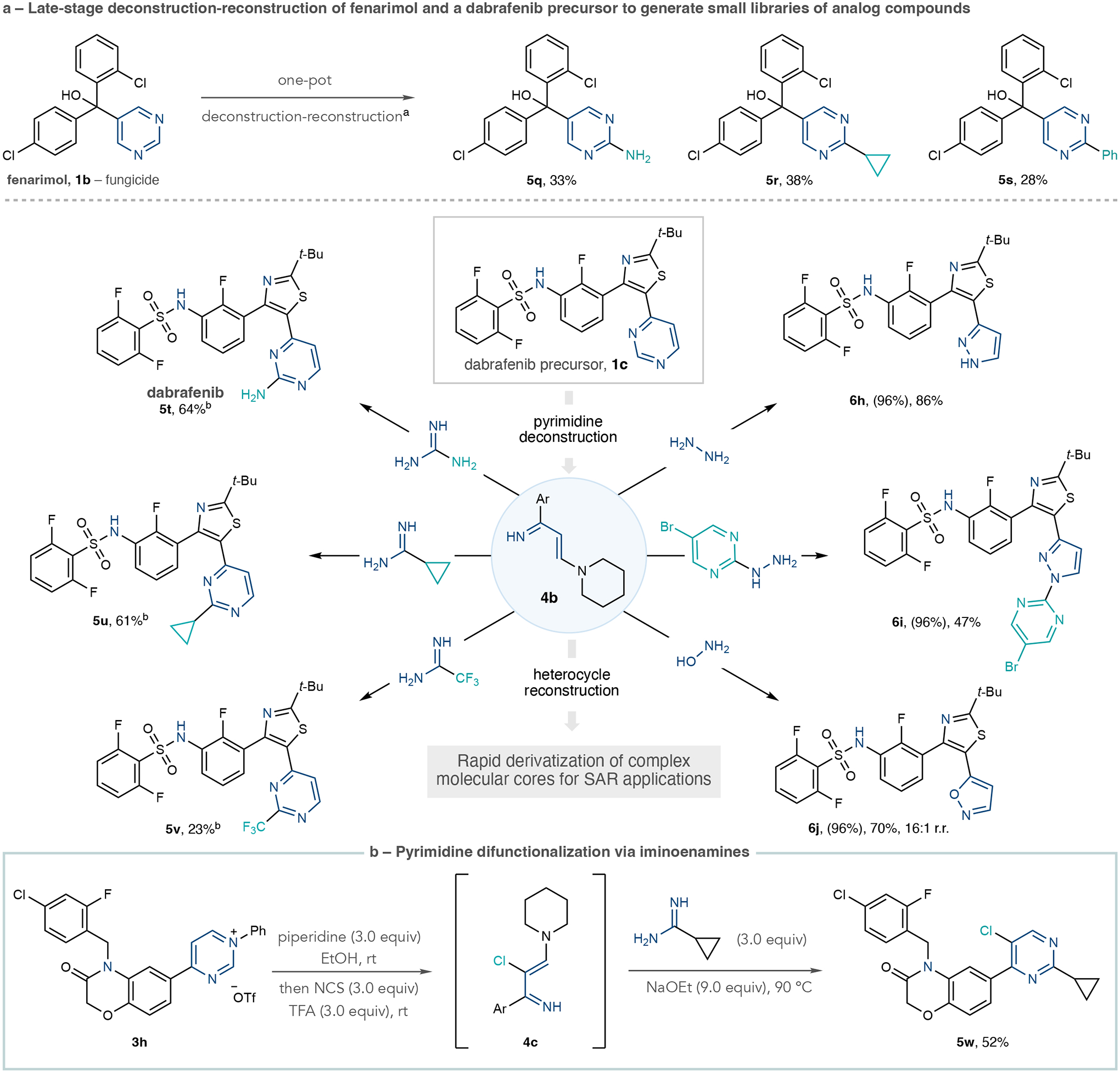
a, Transformations of fenarimol and a dabrafenib precursor. b, Pyrimidine halogenation and difunctionalization. Isolated yields are shown. Yields in parentheses are for pyrimidinium salts. aOne-pot protocol A: Tf2O (1.0 equiv.), 4-nitroaniline (1.0 equiv.), collidine (1.0 equiv.), EtOAc, −78 °C to room temperature. Solvent exchange to EtOH, then piperidine (3.0 equiv.) at room temperature, then amidine (10–20 equiv.), NaOEt (5.0 equiv.). bOne-pot protocol B: same as protocol A except using aniline (1.0 equiv.), amidine (3.0 equiv.) at 70 °C and pyrimidinium salt formation conducted in CH2Cl2. NCS, N-chlorosuccinimide; TFA, trifluoroacetic acid.
