Fig. 4 |. Pyrimidine to pyridine conversions through deconstruction– reconstruction.
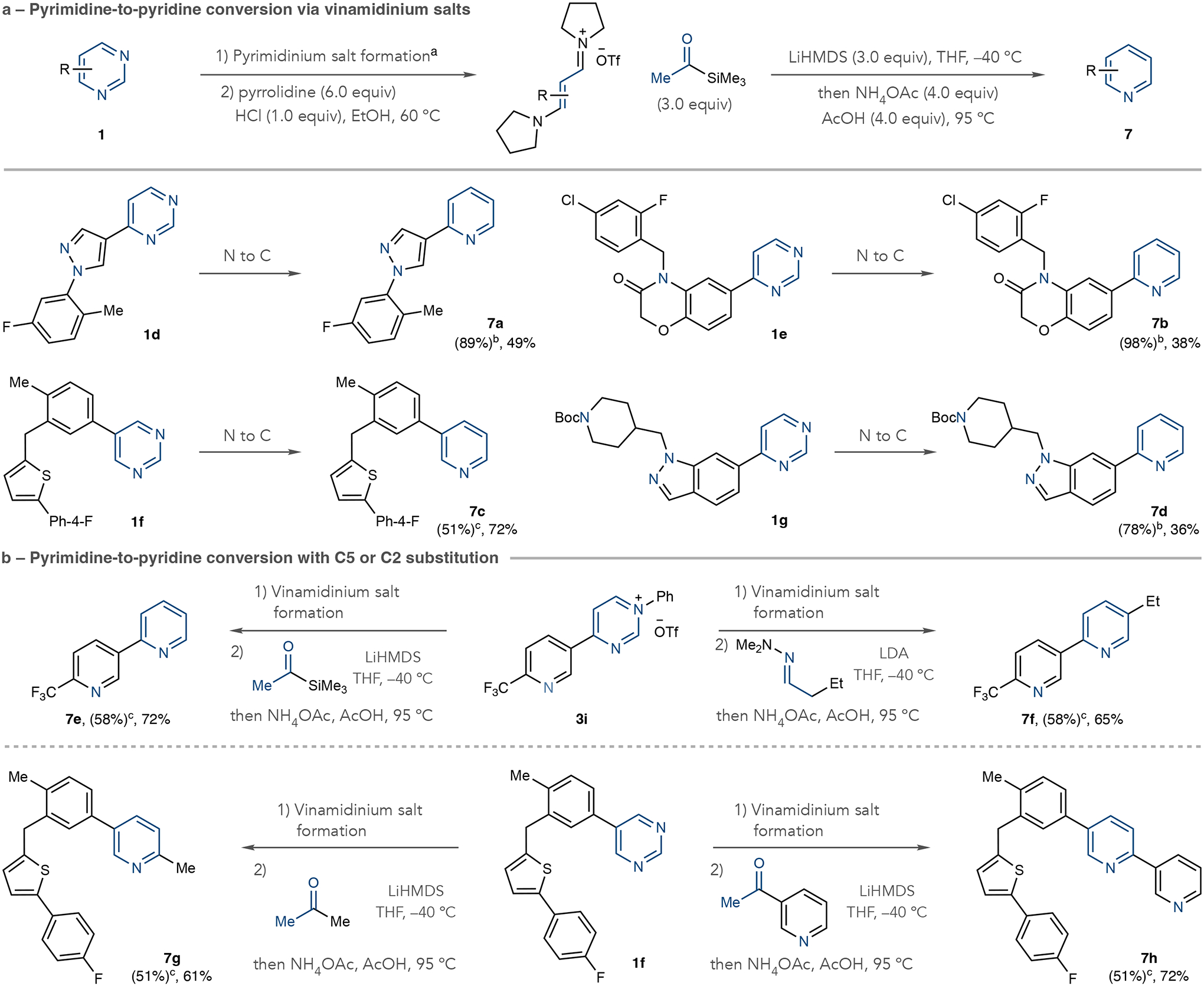
a, Transformations using acetyltrimethylsilane as a nucleophile. b, Pyridine formation using hydrazones or methyl ketones resulting in C5 or C2 substitution. Isolated yields are shown. aPyrimidinium salt formation: Tf2O (1.0 equiv.), aniline (1.0 equiv.), collidine (1.0 equiv.), EtOAc, −78 °C to room temperature. bIsolated yields of pyrimidinium salts. Crude vinamidinium salts were used in the recyclization step. cIsolated yields of vinamidinium salts either directly from pyrimidines or pyrimidinium salts. LiHMDS, lithium hexamethyldisilazide; LDA, lithium diisopropylamine; THF, tetrahydrofuran.
