Fig. 5 |. Computational studies of pyrimidine ring-opening.
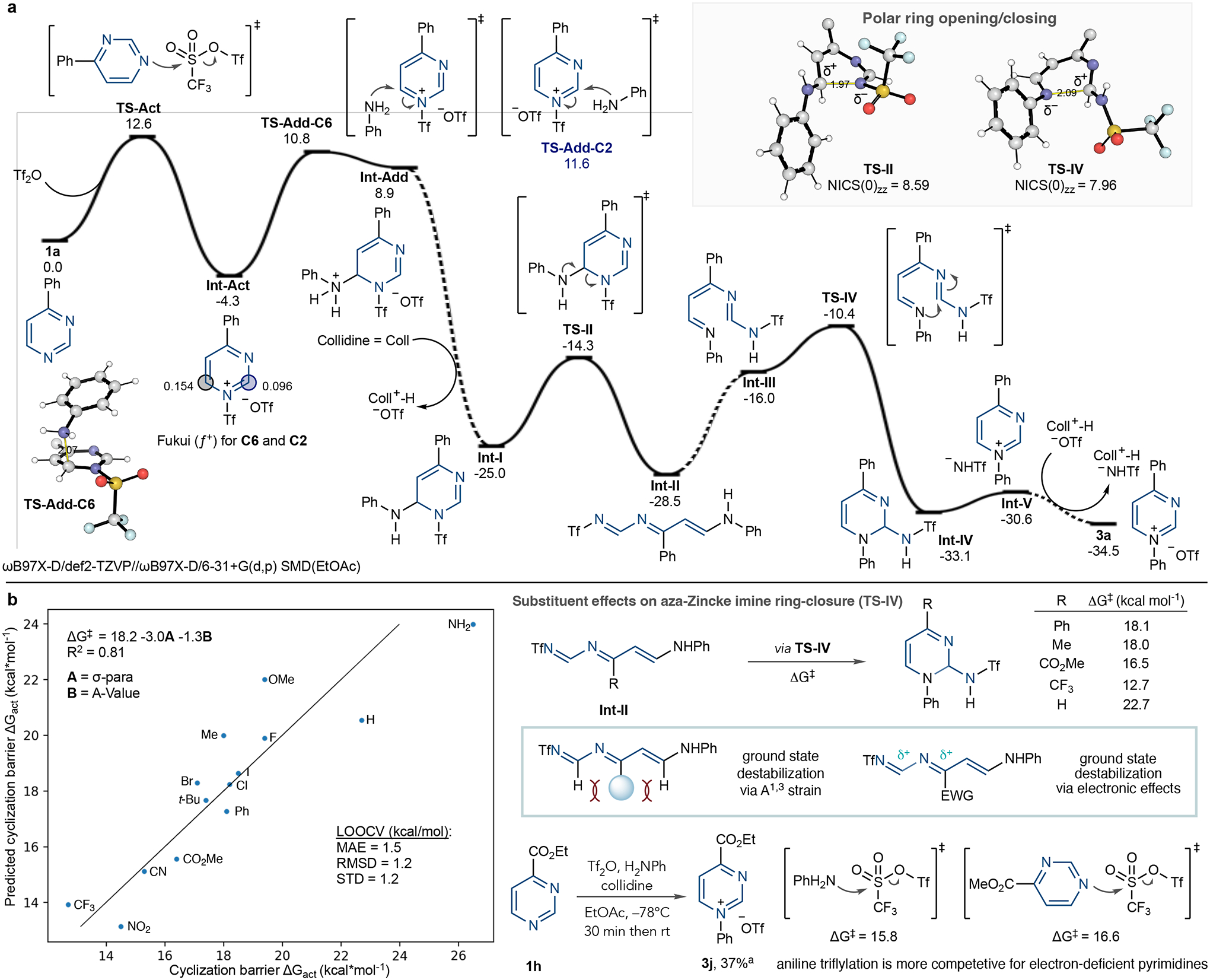
a, Quantum chemical computed reaction mechanism between pyrimidine 3a, Tf2O and aniline; relative Gibbs energies (195.15 K, 1 mol l−1) in kcal mol−1. b, Computational study of the steric and electronic effect of substituents on the cyclization of Int-II and NTf-pyrimidinium salt formation. a1H NMR yield reported. LOOCV, leave-one-out cross-validation; MAE, mean absolute error; r.m.s.d., root mean square deviation; s.d., standard deviation.
