Extended Data Fig. 1 |. Additional examples of pyrimidine functionalization and pyrimidine to pyridine conversion.
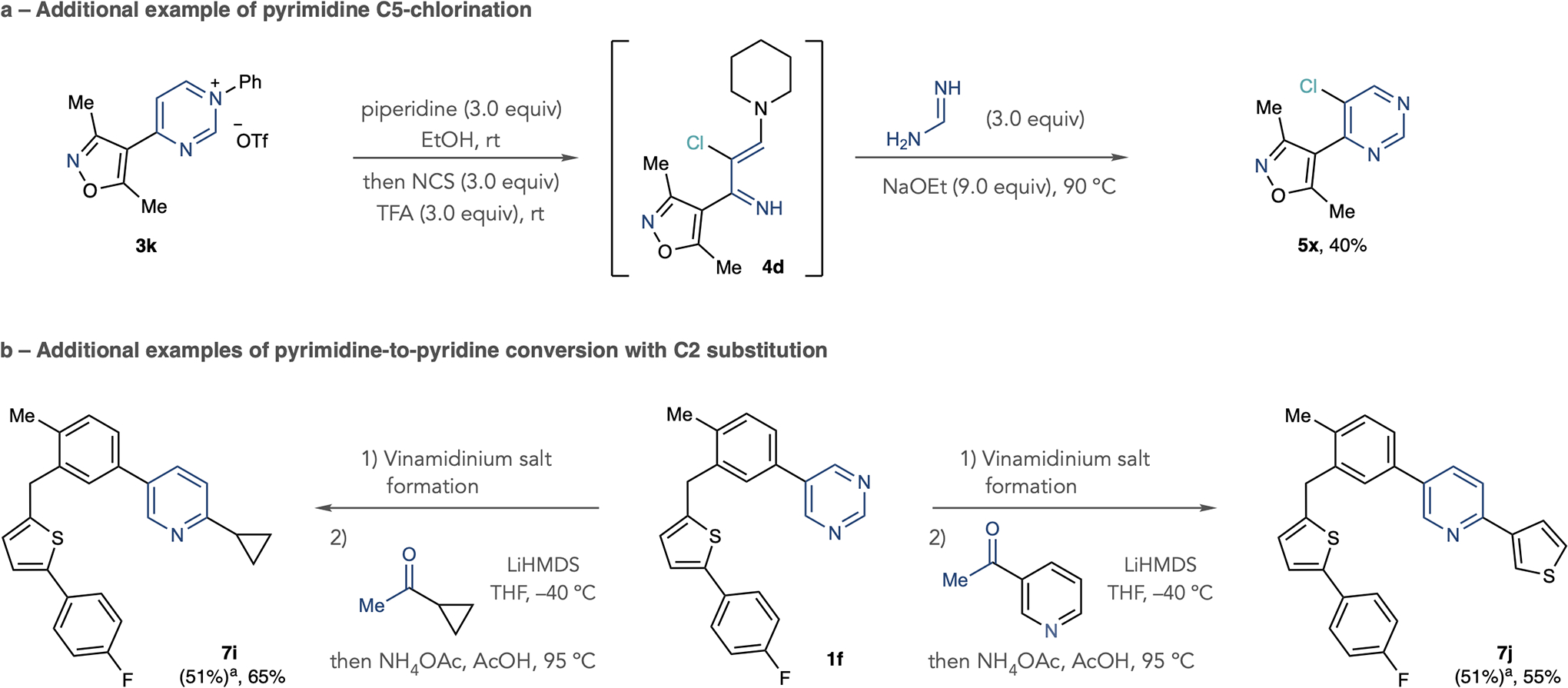
a, Additional example of pyrimidine halogenation. b, Additional examples of pyrimidine to pyridine conversion using methyl ketones. Isolated yields are shown. Vinamidinium salt formation: Tf2O (1 equiv), 4-trifluoromethylaniline (1 equiv), collidine (1 equiv), EtOAc, −78 °C to room temperature, then pyrrolidine (6 equiv), EtOH, 60 °C. aIsolated yield of vinamidinium salt from pyrimidine.
