Abstract
The swine pathogen Actinobacillus pleuropneumoniae serotype 1 was investigated for its ability to adhere to swine, rat, and human buccal epithelial cells (BEC). The highest number of bacteria adhered was to swine BEC. This binding ability was affected by heating, extreme pH, treatment with sodium dodecyl sulfate, ethylenediamine tetraacetate, or periodate, and proteolysis, suggesting that cell-surface glycoproteins participate in adherence and that adherence is based mostly on ionic interactions. Mannose and swine fibronectin may play a direct role in this interaction. Convalescent-phase serum from naturally infected pigs inhibited the adhesion. There was a correlation between bacterial pathogenicity as well as host specificity and the capacity for adherence to swine BEC. Adhesion to swine BEC provides a convenient method to study in vitro the adherence of A. pleuropneumoniae and other pathogens of the pig respiratory tract.
Résumé
Le potentiel d’adhérence du pathogène porcin Actinobacillus pleuropneumoniae sérotype 1 aux cellules épithéliales buccales (BEC) de porc, de rat et d’humain a été étudié. Le plus grand nombre de bactéries a été retrouvé sur les BEC de porc. Ce potentiel d’adhérence était affecté par la chaleur, les valeurs de pH extrêmes, un traitement au sulfate de dodecyl sodium, à l’éthylenediamine tétra-acétate, ou du périodate, et une protéolyse, ce qui suggère que les glycoprotéines de la surface cellulaire participent dans l’adhérence et que l’adhérence est fonction principalement des forces ioniques. Le mannose et la fibronectine d’origine porcine pourraient jouer un rôle direct dans cette interaction. Du sérum provenant de porcs convalescents infectés naturellement inhibait cette adhésion. Il y avait une corrélation entre la pathogénicité bactérienne de même que la spécificité d’hôte et la capacité à adhérer au BEC de porc. L’adhésion aux BEC porcines fournie une méthode pratique pour étudier l’adhérence in vitro d’A. pleuropneumoniae et des autres pathogènes du système respiratoire porcin.
(Traduit par Docteur Serge Messier)
Introduction
Mucosal pathogens initiate disease by adhering to and colonizing the epithelial cell surface (1,2). Adhesins possess typical binding characteristics that contribute to the pathogen’s host specificity and tropism. Some microbial surface components recognizing extracellular matrix (ECM) molecules adhere to substrates with a restricted tissue distribution. Microbial adhesion to these ECM ligands may correlate with the tissue tropism seen in infections (3,4). One of the ECM ligands is fibronectin, a multifunctional adhesive glycoprotein also found on cell surfaces and in body fluids such as plasma.
Actinobacillus pleuropneumoniae is the causative agent of porcine pleuropneumonia. Among its virulence factors are lipopolysaccharide (LPS), capsular polysaccharide, exotoxins, and proteases (5,6). Some virulence factors of this bacterium are released into membrane vesicles (7). Carrier pigs maintain the bacteria in their upper respiratory tract, which suggests that there is a mechanism to attach the bacteria to the nasopharyngeal epithelium.
Oral and nasal cavities are anatomically related. Lymphoid tissues, such as palatine or lingual lymphoid tissue and tonsils, are strategically positioned at the beginning of the digestive and respiratory tracts; therefore, buccal epithelial cells (BEC) have been used to study the adhesion of bacteria, including those of the respiratory tract (8–11). Adherence of A. pleuropneumoniae to several pig respiratory-tract tissues has been reported (12–16), and, although it might be a first step in pleuropneumonia, this adherence continues to be poorly understood. No study has been done on the adherence of this organism to swine BEC, and thus no putative adhesive factors or cell receptors have been characterized.
We evaluated the adherence of A. pleuropneumoniae serotype 1 to BEC of different origins and compared the adherence properties with those of closely related bacteria. We also explored the nature of the factors involved in the adhesion.
Materials and methods
Bacterial strains and growth conditions
We used the following bacterial strains: A. pleuropneumoniae S4074 reference strain of serotype 1 (donated by E.M. Kamp, ID-DLO, Department of Bacteriology, Lelystad, The Netherlands); A. pleuropneumoniae serotype 1, strains 1a, 1b, and 1c (recent isolates from slaughterhouse pigs); A. minor, strain Amy 2B, and A. porcinus (donated by M. Gottschalk, GREMIP, Faculté de médecine vétérinaire, Université de Montréal, Montreal, Quebec); A. suis, A. seminis ATCC 15768, Haemophilus parasuis serotype 5, and Escherichia coli K12 AB1157 (from our collection) (17); Pasteurella multocida (2 isolates from field pigs that had died of bronchopneumonia) (18); and Mannheimia (Pasteurella) haemolytica M16 (microbiota) and OV20 (pathogen) (2 isolates from field ovine). All strains were grown at 37°C in brain–heart infusion broth. In addition, A. pleuropneumoniae, A. porcinus, A. minor, and H. parasuis were supplemented with 10 μg/mL of β-nicotinamide adenine dinucleotide (NAD), and H. parasuis was supplemented with 10% bovine serum as well.
Animals
All animals were kept according to the standard rules for animal care at the Centro de Investigación y de Estudios Avanzados del IPN animal house, in Mexico City. To obtain BEC, we used 14 conventional Yorkshire swine from different litters that were 4 to 8 wk old and, to obtain BEC for some experiments and antibodies, 2 adult pigs. All the pigs were free of A. pleuropneumoniae, Mycoplasma hyopneumoniae, and H. parasuis. We also used 20 male Wistar rats from different litters, weighing between 180 and 200 g, to obtain BEC, and we used 4 female New Zealand rabbits 2 mo old to obtain polyclonal antisera. All the animals were fed ad libitum a commercial, antibioticfree, appropriate diet.
Enzymes and reagents
Proteinase K, trypsin, buffers, detergents, NAD, pig plasma fibrinogen, and other reagents were obtained from Sigma Chemical Company, St. Louis, Missouri, USA.
Adherence assays
Bacteria from overnight cultures were incubated at 37°C under agitation (37.5 × g) until a concentration of 108 colony-forming units (CFU)/mL was reached. Epithelial cells from the cheek internal mucosa of healthy adult humans, rats, swine, and the piglets were obtained as described (10), except the cells were not treated with trypsin and were suspended in 0.1 M Tris-HCl, pH 7.2. Cell viability was checked by trypan-blue exclusion staining at the beginning and at the end of the experiments; it was found to be 95% and 75%, respectively.
For adhesion assays, 5 × 104 cells were incubated with 2 × 108 CFU in a total volume of 1.2 mL for 1 h at 37°C on a rotary shaker (37.5 × g). Then they were washed 3 times with Tris-HCl, pH 7.2, by centrifugation (150 × g for 10 min) to free the cells of nonspecifically attached bacteria. After the first washing, 2 drops of 0.4% erythrosine B were added. An aliquot of the final suspension was air-dried on a glass slide and stained with 0.4% methylene blue. The slide was rinsed in water and examined with a light microscope (Nikon Eclipse E600; Nihon Kohden, Tokyo, Japan). The number of bacteria bound to each of 50 BEC was recorded. At least triplicate measurements were made for each determination, and all counts were done by the same person and verified by 4 other workers. To confirm our results with direct counts, we used the public-domain software program Scion Imagerelease, beta 3b; the number of bacteria adhered was determined from particle size and density counting.
Effect of different treatments on bacterial adherence
The nature of A. pleuropneumoniae S4074 and swine BEC factors involved in adherence was determined through the following treatments of both types of cells: heating to 40°C, 50°C, and 60°C, for 25 min, vigorous agitation (vortex, 5 min), different pHs (3 to 11, 1 h incubation at 37°C with 100 mM Tris, phosphate-buffered saline, or HEPES), and proteolytic enzymes (1 mg/mL of proteinase K and 2.5 μg/mL of trypsin, at 37°C for 10 min). The next treatments were done at pH 7.0 and 37°C with 1 h of incubation: detergents [0.01% (w/v) sodium dodecyl sulfate (SDS), 0.01% (v/v) Tween 20, and Triton X-100], other chemicals [100 mM ethylenediamine tetraacetate (EDTA), 1M NaCl, and 100 mM sodium periodate], carbohydrates (1 mg/mL of heparin, 100 mM N-acetyl-D-galactosamine, N-acetyl-D-glucosamine, glucose, galactose, and mannose), and proteins (30 μg/mL of human and swine fibronectin and 8 μg/mL of swine fibrinogen). All sera [from naturally infected pigs, from a pig vaccinated with a bacterin, and from a pig experimentally infected (all of them of serotype 1), rabbit anti-pig and anti-human fibronectin, and preimmune serum from rabbit and pig] were diluted 1:100.
Fibronectin and antifibronectin antibodies
Swine and human fibronectins from plasma were obtained as previously described (19) and compared with Sigma fibronectin by means of 5% SDS–polyacrylamide gel electrophoresis. Anti-swine and anti-human fibronectin antibodies were induced in rabbits by subcutaneous injection of 200 μg of protein with complete Freund’s adjuvant and 2 boosts of 200 μg each with incomplete Freund’s adjuvant. Antibodies were purified in a Sepharose 4B (Amersham-Pharmacia Biotech, Piscataway, New Jersey, USA)–fibronectin column. Elution was done with 3 M KI. Cross-reaction between human and swine fibronectin was detected by Western blot testing with antihuman and anti-pig fibronectin polyclonal serum, and the titre was obtained by enzyme-linked immunosorbent assay. To visualize fibronectin on the cell surface, we used rabbit anti-swine fibronectin serum as the primary antibody and anti-rabbit IgG labelled with fluorescein isothiocyanate (FITC) as the secondary antibody.
Electron microscopy
Adhesion of A. pleuropneumoniae to fibronectin fibres and networks was done by adsorbing fibronectin onto carbon–Formvar-coated 200-mesh copper grids for 10 min at 25°C and then adding 10 μL of culture (106 CFU/mL). The grids were incubated for 1 h, washed, negatively stained with 1% uranyl acetate for 0.5 min, washed again, and examined with a JEM 1000 electron microscope (Japan Electron Optics Laboratory [JEOL]) at 80 kV.
Statistical analysis
Adhesion assay data were expressed as means ± standard error. Student’s t-test was used to establish statistical significance among treatments. The assays were highly reproducible, the mean numbers of adhered bacteria per BEC for a given bacterial strain not differing significantly through all the experiments (P = 0.05).
Results
Adherence of A. pleuropneumoniae serotype 1 to BEC from various animals
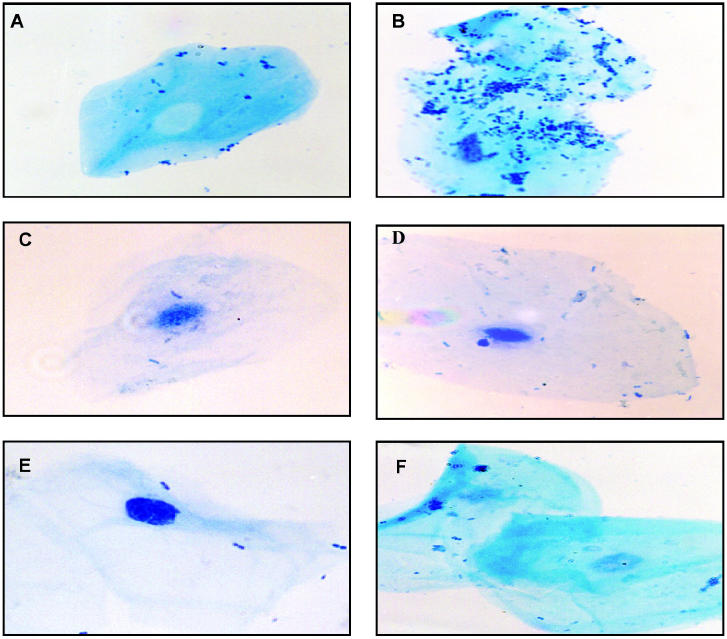
As the results in pigs and piglets were very similar, all the reported experiments were done with piglets, which are easier to manipulate. The specificity of the adherence is shown in Figure 1. Bacterial cells adhered to swine, rat, and human BEC (Figures 1B, D, and F, respectively), swine BEC always showing the highest number of bound bacteria and human BEC the lowest; bacteria were dispersed on the cell surface (Figure 1B).
Figure 1.
Actinobacillus pleuropneumoniae serotype 1 adherence to buccal epithelial cells (BEC) after incubation of 5 × 104 BEC from swine, rats, and humans (B, D, and F, respectively) with 2 × 108 colony-forming units of bacteria. A, C, and E are controls from indigenous flora on swine, rat, and human BEC, respectively. Cells were visualized by light microscopy (original magnification, X400).
Trypsin effect on adherence to swine BEC and to fibronectin
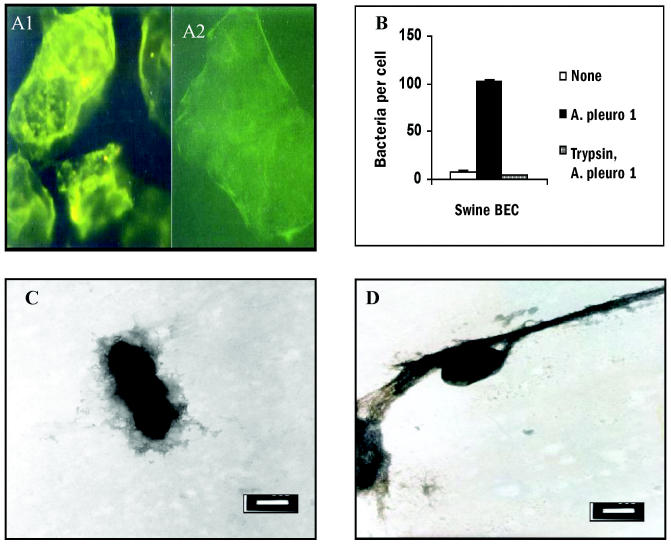
Trypsin’s effect on swine BEC is displayed in Figures 2A and 2B. Figure 2A1 shows the cell fibronectin verified with anti-swine fibronectin antibodies and then the FITC-labelled secondary antibody. The enzyme almost eliminated the fibronectin on the cell surface (Figure 2A2). Trypsin treatment prevented the A. pleuropneumoniae from binding to swine BEC (Figure 2B). Bacteria adhered instantly and strongly to fibronectin fibres, often through the organism’s longitudinal body (Figure 2C). In the fibronectin networks, bacteria were observed covered by the protein (Figure 2D).
Figure 2.
A1: Swine buccal epithelial cells (BEC) incubated with rabbit anti-swine fibronectin polyclonal antibody and then with anti-rabbit immunoglobulin (Ig)G labelled with fluorescein isothiocyanate (FITC). A2: Effect of trypsin on swine BEC surface; cells were incubated with trypsin (2.5 μg/mL) for 10 min, washed with 0.1 M Tris-HCl, pH 7.2, and treated with the same serum as in A1. B: Numbers of bacteria adhering to swine BEC and to trypsin-treated cells. Assay was performed as in Figure 1. None — indigenous flora; A. pleuro 1 — A. pleuropneumoniae serotype 1. C: Electron micrograph of A. pleuropneumoniae incubated for 5 min with fibronectin and stained with uranyl acetate (bar = 500 nm); bacteria are in close interaction with fibronectin networks, covered by the protein. D: Similar interaction of A. pleuropneumoniae with a fibronectin fibre (bar = 500 nm).
Effect of various treatments on adherence to swine BEC
To characterize the A. pleuropneumoniae adhesion factor for swine BEC, the effect of physical and chemical treatments on BEC or on bacteria was tested. The main results are shown in Table I and Figure 3.
Table I.
Effect of various treatments on Actinobacillus pleuropneumoniae adhesion to swine buccal epithelial cells (BEC)
| Mean number of bacteria per cell ± standard error (and % inhibition of adherence)a |
||
|---|---|---|
| Treatment | Bacteria treated | BEC treated |
| Noneb | 92.3 ± 5.8 (0) | 92.3 ± 5.8 (0) |
| Detergentsb (0.01%) | ||
| Sodium dodecyl sulfate | 16.4 ± 3.6 (82.2) | Cellular damage |
| Tween 20 | 63.7 ± 3.8 (30.9) | 30.2 ± 3.0 (67.5) |
| Triton X-100 | 61.1 ± 2.9 (33.8) | 60.7 ± 5.2 (34.2) |
| Chemicalsb | ||
| Ethylenediamine tetraacetate (100 mM) | 42.3 ± 3.6 (54.1) | 80.1 ± 3.7 (13.3) |
| NaCl (1M) | 21.3 ± 4.3 (76.9) | 20.8 ± 3.0 (80.7) |
| Sodium periodate (100 mM) | 34.1 ± 5.4 (63.1)c | 32.8 ± 6.1 (64.5) |
| Proteinsb | ||
| Swine fibronectin (30 μg/mL) | 21.2 ± 3.9 (76.8) | 72.5 ± 6.2 (21.5) |
| Swine fibrinogen (8 μg/mL) | 30 ± 5.3 (67.3) | 24 ± 4.6 (74.0) |
| Enzymesd | ||
| Proteinase K (1 mg/mL) | 37.6 ± 5.2 (59.3) | 15.7 ± 4.6 (82.9) |
| Trypsin (2.5 μg/mL) | 42.6 ± 4.8 (53.8) | 14.5 ± 5.7 (84.3) |
| Trypsin + periodate (100 mM) | 9.7 ± 4.9 (89.5) | 8.4 ± 5.7 (90.9) |
| Carbohydratesb | ||
| D-glucose (100 mM) | 69.8 ± 4.6 (24.4) | 64.9 ± 5.4 (29.7) |
| D-mannose (100 mM) | 12.9 ± 4.2 (86.0) | 13.7 ± 3.6 (85.2) |
| N-acetyl-D-glucosamine (100 mM) | 60.3 ± 4.6 (39.7) | 17.0 ± 5.2 (83.0) |
| N-acetyl-D-galactosamine (100 mM) | 49.3 ± 5.0 (50.7) | 17.3 ± 4.3 (82.7) |
| Heparin (1 mg/mL) | 91.4 ± 6.5 (0.9) | 21.3 ± 2.7 (76.9) |
| Antibodiese (1:100) | ||
| Convalescent-phase pig serumf | 17.2 ± 6.1 (81.4) | 24.2 ± 5.7 (73.8) |
| Pig anti-A. pleuropneumoniaeg | 27.5 ± 8.2 (70.2) | 31.4 ± 7.8 (65.9) |
| Rabbit anti-A. pleuropneumoniae | 16.4 ± 7.8 (82.3) | 28.9 ± 7.9 (68.7) |
| Rabbit anti-pig fibronectin | 78.9 ± 6.8 (14.5) | 21.2 ± 3.3 (76.8) |
| Preimmune pig serum | 61.3 ± 7.2 (33.5) | 68.9 ± 8.5 (25.3) |
| Preimmune rabbit serum | 53.7 ± 5.9 (41.8) | 66.7 ± 5.6 (27.7) |
Assays were performed as described in the legend of Figure 1, and the numbers of indigenous flora were subtracted
At 37°C, pH 7.0, for 1 h
Bacterial clumps
At 37°C for 10 min
Serum was heated at 56°C
From naturally infected pigs
Pigs were inoculated with a commercial bacterin
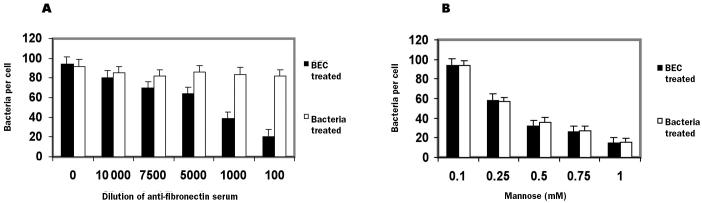
Figure 3.
Concentration effect of anti-pig fibronectin antibodies (A) or mannose (B) on the adherence of A. pleuropneumonie serotype 1 to swine BEC. Assays were performed as in Figure 1.
Heat, vortex agitation, or extreme pH considerably diminished bacterial adherence (approximately 80%). The only detergent that significantly decreased binding (P < 0.001) was SDS. Bacteria exposed to heparin conserved their adherence, but BEC were affected (P < 0.001) (data not shown).
Proteolytic enzymes, such as proteinase K and trypsin, applied to swine BEC reduced adhesion by more than 80% and applied to bacteria reduced adhesion by about 55% to 60%, indicating that cell-surface proteins of both types of cells are crucial for adherence. The addition of trypsin plus periodate to bacteria or swine BEC practically abolished adhesion, reducing it by more than 90%; thus, glycoproteins are involved as recognizing molecules. Because a mannose concentration-dependent effect was found for bacteria and swine BEC (Figure 3B), this carbohydrate could be part of the A. pleuropneumoniae adhesin and BEC receptor structures. Glucose and galactose showed similar behaviour for bacteria and BEC, inhibiting less than 30%. However, N-acetyl-D-galactosamine and N-acetyl-D-glucosamine inhibited more than 80% of BEC.
When A. pleuropneumoniae cells were incubated with pig or human fibronectin or with pig fibrinogen before being exposed to the BEC, these proteins inhibited adhesion 77%, 76%, and 67%, respectively, suggesting a strong interaction with the bacterial surface. Fibrinogen also prevented bacterial binding when the BEC were treated. Furthermore, incubating the swine BEC with either anti-pig or antihuman fibronectin before interaction with the bacteria led to an important decrease in bacterial adherence, suggesting that both fibronectins possess conserved domains that A. pleuropneumoniae recognizes. To test the antibodies’ effect in blocking bacteria–fibronectin binding, an inhibition experiment was performed in which the swine BEC were treated with dilutions of the anti-swine fibronectin serum before interaction with the bacteria; there was a dilution-dependent effect on adhesion to BEC (Figure 3A), confirming the important role of this ECM glycoprotein in adhesion.
Rabbit anti-A. pleuropneumoniae serum or pleuropneumonia convalescent-phase pig serum applied to bacteria decreased attachment significantly (P < 0.001), indicating the importance of the immune system in avoiding the microbial attack. Application of these types of serum to BEC also reduced adherence. Preimmune serum also diminished adherence, but not significantly.
Adhesion of related gram-negative bacteria
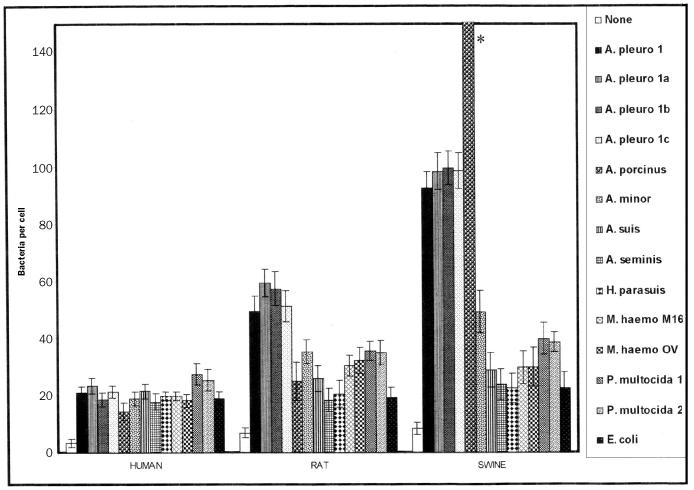
Figure 4 shows the adherence values of human, rat, and swine BEC for 3 A. pleuropneumoniae isolates from slaughterhouse pigs and for different gram-negative species, most in the Pasteurellaceae family. For swine BEC, surprisingly, we found that A. porcinus adherence was 3 times that of the A. pleuropneumoniae reference strain and the 3 isolates. However, A. minor adhered approximately half as much as A. pleuropneumoniae did. With rat BEC, the adherence of A. pleuropneumoniae was approximately 60% of that seen with swine BEC; the adherence of A. porcinus and A. minor to rat BEC was even lower. These 3 Actinobacillus species adhered poorly to human cells. However, A. suis, A. seminis, H. parasuis, and E. coli did not discriminate among the 3 types of cells and in all cases adhered in very low numbers. Both M. haemolytica and P. multocida adhered in low and similar numbers (30% to 40%) to swine and rat BEC and in even lower numbers to human cells, although P. multocida adhered slightly more to human cells than did M. haemolytica.
Figure 4.
Adhesion of 3 A. pleuropneumoniae isolates and related gram-negative bacteria to human, rat, and swine BEC. Assays were done as in Figure 1. None — indigenous flora; A. pleuro 1, 1a, 1b, and 1c — A. pleuropneumoniae serotype 1 reference strain S4074 and 3 isolates; A. porcinus — Actinobacillus porcinus [* adhesion value of 350 ± 13 (standard error)]; A. minor — Actinobacillus minor Amy 2B; A. suis — Actinobacillus suis; A. seminis — Actinobacillus seminis ATCC 15768; H. parasuis — Haemophilus parasuis serotype 5; M. haemo — Mannheimia (Pasteurella) haemolytica M16 (microbiota) and OV20 (pathogen) (2 isolates from field ovine); P. multocida 1 and 2 — Pasteurella multocida (pig pathogen isolates); E. coli — Escherichia coli K12 AB1157.
Discussion
We examined the ability of A. pleuropneumoniae to adhere to BEC. This assay is easy to perform and has reproducible results; thus, it could be used to study the factors involved in adhesion of swine respiratory bacteria. Although A. pleuropneumoniae is species specific, rats and mice can be infected with a high dose (20). We found a higher A. pleuropneumoniae adhesion value for swine BEC than for rat or human cells. Under iron-restricted conditions, this pathogen is able to use porcine but not bovine or human transferrin as an iron source, which suggests that this porcine molecule gives the host specificity to A. pleuropneumoniae (21). Our results indicate that perhaps other host components, such as pig cell receptors, are contributing to the specificity. Probably rat and human BEC do not have receptors for A. pleuropneumoniae recognition. The 3 A. pleuropneumoniae isolates from slaughterhouse pigs of serotype 1, the most virulent and commonly reported in field cases in Mexico, were as adhesive as the reference strain, suggesting that the pigs could have been chronically infected.
Pathogens are likely to have more than 1 adhesin and are therefore able to interact with several receptors and their attendant signaltransduction systems; thus, the attachment of A. pleuropneumoniae to BEC may be mediated by several products. Our finding of inhibition by bacterial agitation in a vortex, which removes and damages fimbriae, outer membrane proteins, membrane vesicles, and capsular polysaccharide, seems to indicate that some or all these structures may be implicated in this phenomenon. Although fimbriae have been demonstrated in A. pleuropneumoniae (22), their role in adhesion has not yet been elucidated. Our demonstration of mannose-dependent adhesion suggests that this carbohydrate could be part of the bacterium adhesin and also of the swine BEC receptor.
Woods and colleagues (23) described the effect of trypsin on the ECM of human BEC, fibronectin being the major component altered. They reported that fibronectin prevented the adherence of Pseudomonas aeruginosa to human BEC and that trypsin treatment promoted attachment. In contrast, Fröman and associates (24) reported that enterotoxigenic strains of E. coli adhered to purified fibronectin and to fibroblast fibronectin fibres of the pericellular matrix (24). Our results with proteolytic enzymes demonstrate that the binding of A. pleuropneumoniae drastically diminishes when swine BEC lose the fibronectin. The 90% inhibition of adherence obtained by treating BEC with periodate plus trypsin confirms that the binding component can be a glycoconjugate, such as fibronectin. In addition, when swine BEC were treated with serum against human or pig fibronectin, the adhesion was also reduced. Fibronectin is a protein found as a component of ECM covered by epithelial or endothelial cells and on tissue cells, such as epithelial cells. In pig lung tissue, fibronectin is widely distributed in both adult and young animals (25). Thus, it must play a significant role in the adherence of many respiratory-tract pathogenic bacteria. When A. pleuropneumoniae was treated with swine or human fibronectin, its adherence to swine BEC decreased, confirming the binding of fibronectin to bacteria and the prevention of further attachment to the BEC. As the inhibition was similar with both fibronectins, probably A. pleuropneumoniae is interacting with shared regions in both proteins, and fibronectin is not the molecule that gives specificity to the binding. Fibronectin could be a ligand connecting BEC receptors to A. pleuropneumoniae. Fibronectin is often bound to integrins, which are cellular receptors (26).
On the other hand, fibrinogen is an abundant plasma protein, and its amounts increase in lung tissues during the pneumonic process. We recently reported the ability of 13 A. pleuropneumoniae serotypes to adhere to this pig protein and the involvement of an outermembrane 60-kDa adhesin in this adherence (27). Thus, fibrinogen could also be participating in the attachment of A. pleuropneumoniae to swine BEC. Divalent ions were also found to be involved in the adherence, as judged by the sensitivity to EDTA, and LPS have been implicated in adherence to other swine cells (28); therefore, the interaction between A. pleuropneumoniae and swine BEC is complex.
To investigate if there is a correlation between virulence and adhesion to swine BEC, we studied some pig commensal and pathogenic bacterial species. Actinobacillus pleuropneumoniae produces acute pleuropneumonia, and in our experiments the reference strain S4074 as well as 3 isolates obtained from slaughterhouse pigs adhered avidly. In contrast, P. multocida, a swine opportunist (18,29), displayed medium adherence to swine BEC. It was also possible to differentiate among the binding abilities of A. pleuropneumoniae and closely related bacteria, some being highly homologous with A. pleuropneumoniae; for example, A. suis adhered 70% less to swine BEC than did A. pleuropneumoniae. Although A. suis can sporadically produce septicemia, endocarditis, and polyarthritis in pigs (30), maybe the oral epithelium is not a good substrate for its attachment; this bacterium adhered in the same numbers to swine and rat cells. Both A. minor and A. porcinus have been reported as part of the normal flora of the pig respiratory tract and oral cavity (31–33); however, Kielstein and coworkers (32) recently reported the isolation of these species from most pigs with pneumonic lesions. In our assay, A. minor attached to swine BEC 50% less and A. porcinus attached in 3 times the numbers, compared with A. pleuropneumoniae. Our results with A. porcinus agree with those of Kielstein and coworkers (33) but contrast with the low pathogenicity reported when A. porcinus was inoculated intranasally and orally in gnotobiotic piglets, although in that experiment the bacterium was reisolated from the brain of an animal showing mild ataxia. This bacterium also has been occasionally isolated from pig brain (34,35). An explanation for the high avidity of A. porcinus to swine BEC could be that the oral route is the preferred portal of entry for this species, although this must be demonstrated. Interestingly, A. porcinus bound in lower numbers than A. pleuropneumoniae to rat and human cells, indicating its high host specificity. Haemophilus parasuis is frequently found in the upper respiratory tract of healthy pigs and in low numbers in the oral cavity; it also showed a low adhesion value (80% lower than A. pleuropneumoniae). On the other hand, A. seminis and M. haemolytica that had not been isolated from pigs showed a very low adherence to swine BEC; 1 of the isolates of M. haemolytica had been obtained from microbiota and the other from a sheep with pneumonia. Escherichia coli is not part of the normal oral flora of swine, rats, and humans; this bacterium showed the same low affinity for the 3 types of cells. Together, these results indicate that adherence to host cells is crucial for both commensal and pathogenic bacteria for colonization; however, pathogenic bacteria show stronger attachment and specificity. The adherence to swine BEC could be a model for studying swine respiratory-tract pathogens.
We have provided evidence that A. pleuropneumoniae can be strongly associated with the epithelium of the swine oral cavity. Oral epithelium may be a vehicle for bacterial deposition in other sites. The attachment of A. pleuropneumoniae to tonsillar epithelial cells may contribute to the bacterium’s establishment in the tonsils (14), being in contact with mouth components and saliva. The binding of A. pleuropneumoniae to BEC may serve at least 2 functions: first, bacteria may attach to BEC as an early step in colonization of the host respiratory tract; second, this binding may be related to the carrier state in animals with chronic infection. It is evident that, in ecologic terms, the oral environment is a perfect niche for some bacteria.
Acknowledgments
The authors thank Dr. V. Tenorio for pig serum and E. Molina and M. Reyes for technical assistance. This project was supported through Conacyt grants 28755B and G38590B. Dr. Hamer-Barrera was supported by a scholarship from Conacyt (53370) and by Universidad Autónoma de Sinaloa.
Footnotes
Dr. Hamer-Barrera’s current address is Facultad de Medicina Veterinaria y Zootecnia, Universidad Autónoma de Sinaloa, Ap. postal 1057, Culiacán, Sinaloa 80000, México.
References
- 1.Beachey EH. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surfaces. J Infect Dis. 1981;143:325–344. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- 2.Johanson WG, Woods DE, Chaudhuri T. Association of respiratory tract colonization with adherence of gram-negative bacilli to epithelial cells. J Infect Dis. 1979;139:667–673. doi: 10.1093/infdis/139.6.667. [DOI] [PubMed] [Google Scholar]
- 3.Patti JM, Allen BL, McGavin MJ, Hook M. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol. 1994;48:585–617. doi: 10.1146/annurev.mi.48.100194.003101. [DOI] [PubMed] [Google Scholar]
- 4.Wann ER, Gurusiddappa S, Hook M. The fibronectin-binding MSCRAMM FnbpA of Staphylococcus aureus is a bifunctional protein that also binds to fibrinogen. J Biol Chem. 2000;275:13863–13871. doi: 10.1074/jbc.275.18.13863. [DOI] [PubMed] [Google Scholar]
- 5.Haesebrouck F, Chiers K, VanOverbeke I, Ducatelle R. Actinobacillus pleuropneumoniae infections in pigs: the role of virulence factors in pathogenesis and protection. Vet Microbiol. 1997;58:239–249. doi: 10.1016/s0378-1135(97)00162-4. [DOI] [PubMed] [Google Scholar]
- 6.Negrete AE, Tenorio VR, Serrano JJ, Garcia CC, de la Garza M. Secreted proteases from Actinobacillus pleuropneumoniae serotype 1 degrade porcine gelatin, hemoglobin and IgA. Can J Vet Res. 1994;58:83–86. [PMC free article] [PubMed] [Google Scholar]
- 7.Negrete AE, García RM, Reyes ME, Godínez D, de la Garza M. Membrane vesicles released by Actinobacillus pleuropneumoniae contain proteases and Apx toxins. FEMS Microbiol Lett. 2000;191:109–113. doi: 10.1111/j.1574-6968.2000.tb09326.x. [DOI] [PubMed] [Google Scholar]
- 8.Courtney HS, Dale JB, Hasty DL. Differential effects of the streptococcal fibronectin-binding protein, FBP54, on adhesion of group A streptococci to human buccal cells and Hep-2 tissue culture cells. Infect Immun. 1996;64:2415–2419. doi: 10.1128/iai.64.7.2415-2419.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kopp PA, Schmitt M, Wellensiek AJ, Blobel H. Isolation and characterization of fibronectin-binding sites of Borrelia garinii N34. Infect Immun. 1995;63:3804–3808. doi: 10.1128/iai.63.10.3804-3808.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaca-Pacheco S, García O, Paniagua G. The lom gene of bacteriophage lambda is involved in Escherichia coli K12 adhesion to human buccal epithelial cells. FEMS Microbiol Lett. 1997;156:129–132. doi: 10.1111/j.1574-6968.1997.tb12717.x. [DOI] [PubMed] [Google Scholar]
- 11.Vaca-Pacheco S, Paniagua G, Garcia O, de la Garza M. The clinically isolated FIZ15 bacteriophage causes lysogenic conversion in Pseudomonas aeruginosa PAO1. Curr Microbiol. 1999;38:239–243. doi: 10.1007/pl00006794. [DOI] [PubMed] [Google Scholar]
- 12.Jacques M, Belanger M, Roy G, Foiry B. Adherence of Actinobacillus pleuropneumoniae to porcine tracheal epithelial cells and frozen lung sections. Vet Microbiol. 1991;27:133–143. doi: 10.1016/0378-1135(91)90004-y. [DOI] [PubMed] [Google Scholar]
- 13.Haesebrouck F, Van de Kerkhof A, Chiers K, Ducatelle R. Interactions of Actinobacillus pleuropneumoniae with alveolar epithelial cells. Proc Congr Eur Soc Vet Pathol, Ghent, Belgium 1996:87.
- 14.Chiers K, Haesebrouck F, Van Overbeke I, Charlier G, Ducatelle R. Early in vivo interactions of Actinobacillus pleuropneumoniae with tonsils of pigs. Vet Microbiol. 1999;68:301–306. doi: 10.1016/s0378-1135(99)00048-6. [DOI] [PubMed] [Google Scholar]
- 15.Dom P, Haesebrouck F, Ducatelle R, Charlier G. In vivo association of Actinobacillus pleuropneumoniae serotype 2 with the respiratory epithelium of pigs. Infect Immun. 1994;62:1262–1267. doi: 10.1128/iai.62.4.1262-1267.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Overbeke I, Chiers K, Charlier G, et al. Characterization of the in vitro adhesion of Actinobacillus pleuropneumoniae to swine alveolar epithelial cells. Vet Microbiol. 2002;88:59–74. doi: 10.1016/s0378-1135(02)00080-9. [DOI] [PubMed] [Google Scholar]
- 17.García CC, Montañez C, Tenorio V, et al. A 24-kDa cloned zinc metalloprotease from Actinobacillus pleuropneumoniae is common to all serotypes and cleaves actin in vitro. Can J Vet Res. 2000;64:88–95. [PMC free article] [PubMed] [Google Scholar]
- 18.Negrete AE, Tenorio VR, de la Garza M. Secretion of proteases from Pasteurella multocida isolates. Curr Microbiol. 1999;38:64–67. doi: 10.1007/pl00006775. [DOI] [PubMed] [Google Scholar]
- 19.Ruoslahti E, Hayman EG, Pierschbacher M, Engvall E. Fibronectin: purification, immunochemical properties and biological activities. Meth Enzymol. 982;82:803–831. doi: 10.1016/0076-6879(82)82103-4. [DOI] [PubMed] [Google Scholar]
- 20.Nakai T, Sawata A, Kume K. Pathogenicity of Haemophilus pleuropneumoniae for laboratory animals and possible role of its hemolysin for production of pleuropneumonia. Jpn J Vet Sci. 1984;46:851–858. doi: 10.1292/jvms1939.46.851. [DOI] [PubMed] [Google Scholar]
- 21.González GC, Caamano DL, Schryvers AB. Identification and characterization of a porcine-specific transferrin receptor in Actinobacillus pleuropneumoniae. Mol Microbiol. 1990;4:1173–1179. doi: 10.1111/j.1365-2958.1990.tb00692.x. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Tennent JM, Ingham A, Beddome G, Prideaux C, Michalski WP. Identification of type 4 fimbriae in Actinobacillus pleuropneumoniae. FEMS Microbiol Lett. 2000;189:15–18. doi: 10.1111/j.1574-6968.2000.tb09199.x. [DOI] [PubMed] [Google Scholar]
- 23.Woods DE, Straus DC, Johanson WG, Bass JA. Role of fibronectin in the prevention of adherence of Pseudomonas aeruginosa to buccal cells. J Infect Dis. 1981;143:784–790. doi: 10.1093/infdis/143.6.784. [DOI] [PubMed] [Google Scholar]
- 24.Fröman G, Switalski LM, Faris A, Wadström T, Höök M. Binding of Escherichia coli to fibronectin. J Biol Chem. 1984;259:14899–14905. [PubMed] [Google Scholar]
- 25.Mills AN, Haaworrth SG. Pattern of connective tissue development in swine pulmonary vasculature by immunolocalization. J Pathol. 1987;153:171–176. doi: 10.1002/path.1711530210. [DOI] [PubMed] [Google Scholar]
- 26.Isberg RR, Tran Van Nhieu G. Binding and internalization of microorganisms by integrin receptors. Trends Microbiol. 1994;2:10–14. doi: 10.1016/0966-842x(94)90338-7. [DOI] [PubMed] [Google Scholar]
- 27.Martínez-Zúñiga R, Enríquez VI, Godinez D, Guerrero AL, de la Garza M. Actinobacillus pleuropneumoniae: adhesion to swine fibrinogen. Proc Int Pateurellaceae Soc Conf, Banff, Alberta 2002:50.
- 28.Paradis S, Dubreuil D, Rioux S, Gottschalk M, Jacques M. High molecular mass lipopolysaccharides are involved in Actinobacillus pleuropneumoniae adherence to porcine respiratory tract cells. Infect Immun. 1994;62:3311–3319. doi: 10.1128/iai.62.8.3311-3319.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ciprián A, Pijoan C, Cruz T, et al. Mycoplasma hyopneumoniae increases the susceptibility of pigs to experimental Pasteurella multocida pneumonia. Can J Vet Res. 1988;52:434–438. [PMC free article] [PubMed] [Google Scholar]
- 30.Sanford SE, Miniats OP. Actinobacillus suis septicemia mimicking erysipelas in sows. Can Vet J. 1988;29:595–598. [PMC free article] [PubMed] [Google Scholar]
- 31.Moller K, Killian M. V factor-dependent members of the family Pateurellaceae in the porcine upper respiratory tract. J Clin Microbiol. 1990;28:2711–2716. doi: 10.1128/jcm.28.12.2711-2716.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kielstein P, Wuthe HH, Angen O, Mutters R, Ahrens P. Phenotypic and genetic characterization of NAD-dependent Pasteurellaceae from the respiratory tract of pigs and their possible pathogenic importance. Vet Microbiol. 2001;81:243–255. doi: 10.1016/s0378-1135(01)00351-0. [DOI] [PubMed] [Google Scholar]
- 33.Chiers K, Haesebrock F, Mateusen B, Van Overbeke I, Ducatelle R. Pathogenicity of Actinobacillus minor, Actinobacillus indolicus and Actinobacillus porcinus strains for gnotobiotic piglets. J Vet Med B. 2001;48:127–131. doi: 10.1046/j.1439-0450.2001.00433.x. [DOI] [PubMed] [Google Scholar]
- 34.Rapp VJ, Gabrielson DA. Prevalence of Haemophilus parasuis serovars among isolates from swine. Am J Vet Res. 1992;53:659–662. [PubMed] [Google Scholar]
- 35.Blackall PJ, McKechnie K, Sharp T. Isolation of Haemophilus taxon D from pigs in Australia. Aust Vet J. 1994;71:262–265. doi: 10.1111/j.1751-0813.1994.tb03426.x. [DOI] [PubMed] [Google Scholar]