Abstract
Early reemergence of consciousness predicts long-term functional recovery for patients with severe brain injuries. However, tools to reliably detect consciousness in the intensive care unit (ICU) are lacking. Transcranial magnetic stimulation-electroencephalography has the potential to detect consciousness in the ICU, predict recovery, and prevent premature withdrawal of life-sustaining therapy.
Keywords: coma, consciousness, complexity, transcranial magnetic stimulation electroencephalography (TMS-EEG)
Introduction
In intensive care units (ICUs) around the world, patients with severe brain injuries lie in bed, unresponsive, connected to life-sustaining ventilators and brain monitors. Within days of injury, clinicians assess each patient’s chances for long-term recovery and provide families with a prognosis upon which decisions about life-sustaining therapy are made. Early recovery of consciousness is a key milestone that predicts long-term functional recovery [1–4]. However, because consciousness (in the sense of having an experience) is inherently subjective [5], it can be difficult to measure operationally and may go unrecognized in some patients [6]. Of the millions of people globally who experience a severe brain injury each year [7, 8], 15–20% may be covertly conscious, with higher levels of consciousness than their bedside behavioral assessment suggests [3, 6]. Either due to pain, sedation, or injury to central and peripheral motor pathways, a conscious individual’s capacity for self-expression may be compromised. In this setting, it is essential that tools are developed to detect signs of consciousness (diagnosis) and provide families with an accurate picture of their loved ones’ chances of functional recovery (prognosis).
To address this challenge, multiple advanced neurotechnologies have been developed over the past two decades, shedding new light on the physiology of consciousness and coma recovery. Yet reliable assessment of consciousness in the ICU remains elusive. Resting-state functional MRI studies reveal brain networks necessary for consciousness [5], but no single network is sufficient [9]. EEG studies demonstrate that coherence and entropy correlate with consciousness [10], but consciousness can emerge across a broad range of EEG spectral patterns [11]. Task-based functional MRI (fMRI) and EEG detect volitional brain activity [3, 4, 6] but suffer from high false-negative rates (i.e., failing to detect command-following in a conscious individual) [6, 12] and are challenging to implement in the ICU.
It is against this historical backdrop that transcranial magnetic stimulation (TMS)-EEG measurement of brain complexity becomes relevant for clinical translation in the ICU (Figure 1). Inspired by theoretical principles [13–15], TMS-EEG gauges, as a proxy for consciousness, the ability of distributed and differentiated groups of neurons to interact as a whole to produce complex dynamics. Growing evidence supports the notion that brain complexity – defined as the coexistence of differentiation and integration in the thalamocortical network – is a reliable marker of consciousness [16, 17]. Repeated administration of a brief TMS pulse to the cerebral cortex triggers a long-lasting (∼300 milliseconds) brain-wide response whose complexity can be measured by EEG and quantified as the perturbational complexity index (PCI) [18]. The PCI value is normalized, with 0 corresponding to the absence of statistically significant EEG response, and 1 being maximally complex.
Figure. 1. Stimulating the brain to determine its capacity for consciousness.
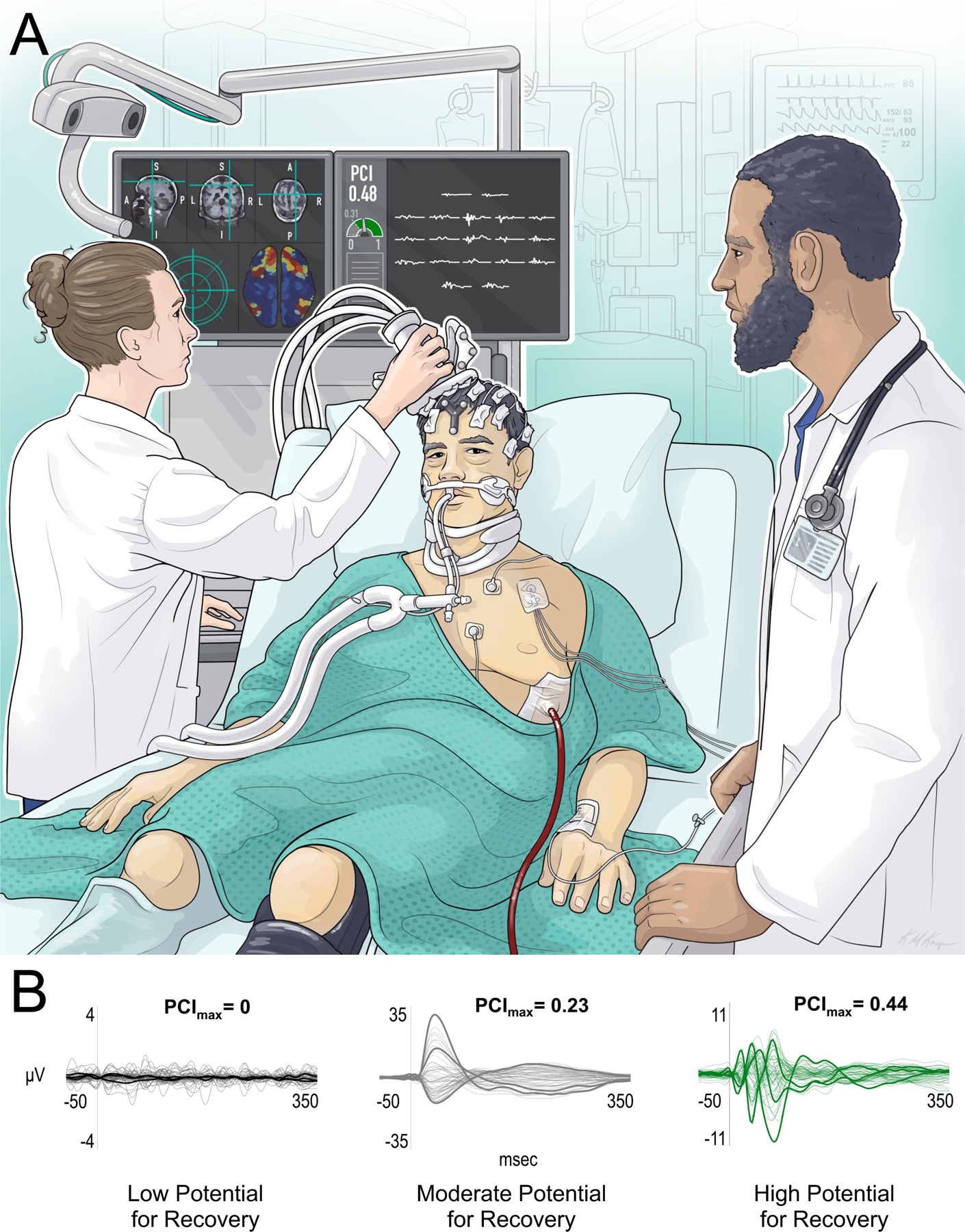
(A) A technologist (left) with expertise in transcranial magnetic stimulation-electroencephalography (TMS-EEG) measures the perturbational complexity index (PCI) in a patient with a severe traumatic brain injury (center), while a physician observes (right). The technician stimulates the right premotor cortex based on precise anatomic guidance provided by the T1-weighted magnetic resonance imaging (MRI) scan on the top of the left screen. A functional MRI connectivity map is used to identify cortical sites to be targeted (e.g., highly connected regions shown in red are likely to have higher PCI values). The turquoise aiming device on the bottom left of the screen provides real-time feedback about the precision of target stimulation. On the right screen, the technologist visually assesses for artifacts and determines the reliability of TMS-evoked potentials recorded by 19 EEG electrodes. In this illustrative patient, the PCI value of 0.48 is above the empirically derived cut-off of 0.31, indicating that the patient’s brain has the same complexity as that of conscious subjects. (B) TMS-evoked EEG waveforms that indicate low (black), moderate (gray), and high (green) potential for recovery of behavioral signs of consciousness. These waveforms are adapted from Casarotto et al. 2016 [19], where low potential for recovery was observed with PCI = 0, moderate potential for recovery with 0 < PCI ≤ 0.31, and high potential for recovery with PCI > 0.31. Abbreviations: A = anterior; I = inferior; L = left; P = posterior; R = right; S = superior.
Over the past 10 years, PCI values above an empirically derived threshold (PCI > 0.31) have identified consciousness with 100% specificity and 100% sensitivity in a validation dataset of 48 conscious brain-injured patients and 102 healthy subjects across a broad range of behavioral states, including resting wakefulness, anesthesia, slow-wave sleep and REM sleep [18, 19]. That is, whenever PCI ≤ 0.31, the subject is either in a deep sleep or anesthetized, while PCI > 0.31 corresponds to full consciousness, REM sleep with report of dreams, or a dissociative state with preserved self-awareness (i.e., ketamine). Furthermore, TMS-EEG measurements of PCI detect high complexity in > 90% of severely brain-injured patients who have recovered behaviorally to a minimally conscious state [19], as compared to a ~60% detection rate with task-based fMRI and EEG motor imagery paradigms [6]. This unparalleled performance motivates the translation of TMS-EEG to the ICU as a tool to measure consciousness – a first, albeit primitive, consciousness-detector [20].
Detecting Signs of Consciousness
TMS-EEG provides four diagnostic advantages over resting-state and task-based methods to assess consciousness. First, TMS-EEG applies a perturbational rather than an observational approach. Unlike resting-state EEG [10, 21], which probes correlational network properties, TMS-EEG measures neuronal interactions from a causal perspective, providing a more reliable (i.e., higher signal-to-noise ratio) and comprehensive assessment of brain dynamics [17]. For example, TMS-EEG may uncover complexity in the presence of diffuse slowing of the resting-state EEG background.
Second, TMS-EEG bypasses sensory systems, which can be impaired in patients with traumatic, hypoxic, and other forms of brain injury. Rather than relying on vision, touch, hearing, or smell, TMS-EEG directly accesses the primary sensory and association regions of the cerebral cortex, reducing confounders and increasing the reliability of the physiological measurements.
Third, TMS-EEG bypasses motor systems and is thus not dependent on a behavioral output. Focal lesions in the brain, spinal cord or peripheral nerves may disrupt central or peripheral motor pathways, preventing self-expression. In addition, diffuse weakness from myopathy or polyneuropathy may prevent self-expression due to quadriplegia or prolonged mechanical ventilation [22]. Patients with extensive trauma may also have pain or orthopedic injuries that prohibit movement, even if neural motor pathways are intact.
Fourth, no cognitive effort is required for TMS-EEG, an advantage that is particularly relevant to patients with orbitofrontal and basal forebrain lesions, which deplete attentional and working memory resources. Patients with frontal-forebrain disconnection syndromes may be unable to sustain attention on simple tasks, leading to delayed or absent responses during task-based paradigms despite preserved consciousness [23].
For these four reasons, TMS-EEG measurements provide unprecedented sensitivity and specificity of the state of consciousness in severely brain-injured patients [18, 19]. While other advanced methods for detecting signs of consciousness, such as task-based EEG and fMRI, are currently more feasible and more likely to be reimbursed by insurers [8, 24], the methodological advantages of TMS-EEG provide a rationale for its potential utility in the ICU, as well as its cost-effectiveness. We advocate for multimodal studies [25] that compare the performance characteristics of TMS-EEG, task-based EEG [3, 4, 6], task-based fMRI [6, 26], and behavioral assessments in critically ill patients with DoC [27–30]. A key unanswered question in the ICU, where patients may not tolerate cessation of sedation for longer than a few minutes, is whether TMS-EEG provides greater diagnostic yield for detecting signs of consciousness than repeated behavioral assessments.
Clinical Utility and Ethical Imperative
The most compelling clinical application of TMS-EEG to the ICU is as a prognostic tool. Because early emergence of consciousness in the ICU predicts long-term outcomes, the unparalleled diagnostic characteristics of TMS-EEG can improve prognostic accuracy. Indeed, emerging evidence from patients in the subacute-to-chronic stage of brain injury suggests that TMS-EEG measurements of PCI can stratify behaviorally unresponsive patients into subgroups with high, moderate, and low chances of recovering behavioral signs of consciousness [19].
The ethical rationale for clinical translation of TMS-EEG into the ICU setting is its potential to improve goal-concordant care and prevent premature withdrawal of life-sustaining therapy (WLST). Currently, WLST is the most common cause of death (~80%) in patients with acute disorders of consciousness (DoC) due to anoxic [31] or traumatic [32] causes. However, families and caregivers often make life-or-death decisions in the ICU without a clear understanding of their loved one’s state of consciousness or potential for recovery. Given that impressions of a patient’s level of consciousness and likelihood of recovery are primary determinants of family decisions about WLST [33], early detection of consciousness in the ICU may have life-or-death consequences.
Ethical principles guiding WLST decisions include autonomy – the importance of respecting and promoting a patient’s personal values and goals – and non-maleficence – the idea that clinicians should not do harm by prolonging life for a patient whose current and future quality-of-life is unacceptable to them [34]. However, there is often insufficient evidence available to determine whether a behaviorally unresponsive patient is conscious or not [35] and to determine their likelihood of recovering consciousness [9, 31]. Obtaining more accurate indicators of consciousness and predictors of recovery is essential for ensuring that ethical, goal-concordant decisions are made in clinical care [36]. To promote patient autonomy and avoid undue harm, decisions about WLST, pain control, and neurorehabilitation should be informed by a patient’s level of consciousness and capacity for recovery – a goal that TMS-EEG, with its unprecedented sensitivity, is ideally poised to accomplish in the ICU.
Importantly, recovery of consciousness is not inextricably linked to recovery of functional independence. Although evidence of consciousness (behavioral or covert) in the ICU predicts long-term functional recovery at the group level [1–4], individual patients with covert consciousness may die after discharge from the ICU or experience long-term functional disability [4]. For example, sensorimotor deficits that impair self-expression in the ICU may persist, leading to chronic functional disability and a compromised quality of life despite preserved consciousness [37]. Future studies will thus have a higher likelihood of impacting clinical practice if they select endpoints that measure recovery of both consciousness and functional independence [38, 39]. In parallel, there is an ethical imperative to elucidate clinician and family perspectives on how advanced neurotechnologies like TMS-EEG should be responsibly integrated into clinical practice [35, 40–42].
Endorsement Without Implementation
In 2018, twelve years after the first report of covert consciousness [43], academic institutions began to endorse the clinical implementation of advanced neurotechnologies for detection of covert consciousness. The American Academy of Neurology, American Congress of Rehabilitation Medicine and the United States National Institute of Disability, Independent Living and Rehabilitation Research endorsed these techniques for the clinical evaluation of patients with DoC in the subacute-to-chronic stages of recovery [44]. In 2020, the European Academy of Neurology similarly recommended task-based fMRI and EEG. They expanded this clinical recommendation to include TMS-EEG and extended the time course to the acute setting [45]. That same year, the International Federation of Clinical Neurophysiology proposed a stepwise clinical evaluation of consciousness [46], endorsing TMS-EEG to identify unresponsive patients who may benefit from rehabilitation.
Yet despite the endorsement of advanced neurotechnologies for clinical use, implementation has stalled. An international survey conducted by the Curing Coma Campaign found that less than 10% of clinicians caring for patients with DoC have access to these tools [8]. The slow pace of clinical implementation may be partly attributable to debates about the suitability of advanced neurotechnologies for clinical use in the ICU [47], given that guideline recommendations are based mostly on subacute-to-chronic data. Moreover, the strength of the evidence is poor due to the limited number of studies and their small sample sizes. Nevertheless, international support is growing for clinical implementation of advanced neurotechnologies [48], consistent with these guidelines. Accordingly, we argue here that the primary barriers to clinical translation are methodological and logistical, and therefore capable of being overcome by a sustained, international effort.
Barriers to Clinical Translation of TMS-EEG
Clinical implementation of TMS-EEG has been limited by multiple methodological hurdles [49, 50]. A 2022 consensus statement identified three inter-connected challenges preventing clinical translation of TMS-EEG: 1) the logistical complexity of its application; 2) the technical difficulty of obtaining high-quality EEG signals; and 3) insufficient validation of analytic pipelines [51]. We advocate for a comprehensive framework to overcome each of these challenges.
From a logistical standpoint, clinical translation of TMS-EEG to the ICU setting will require a portable tool. TMS equipment is comprised of a TMS stimulator and coil, a cooling unit to prevent overheating of the system, a navigation system with a stereotactic camera to guide precise cortical targeting [52], and a computer display with a three-dimensional brain map for real-time monitoring (Figure 1). While several companies have reduced the dimensions of the TMS unit, few have created a compact, portable TMS device that can feasibly be used in an ICU patient’s room.
To maximize feasibility and deployability, a portable TMS-EEG device will need to support a variety of inputs to its neuroimaging-guided navigation system. At hospitals with access to advanced MRI data (e.g., resting-state fMRI for functional connectivity or diffusion MRI for structural connectivity), a TMS-EEG technologist may upload connectivity maps to the TMS-EEG console and view connectivity data interactively (Figure). At hospitals without access to advanced MRI data, the technologist may use standard neuroimaging guidance with computed tomography or T1-weighted MRI scans [18, 19, 53]. Of note, recent advances in machine learning enable synthesis of T1-weighted MRI scans at 1 mm isotropic resolution from low-resolution MRI scans, or even from CT scans [54, 55]. These advances have potential to support international dissemination of imaging-guided TMS-EEG at hospitals in academic and community settings.
From a technical standpoint, optimizing EEG signal-to-noise properties is essential to obtain reliable PCI measurements and reduce the duration of the TMS-EEG session. Real-time read-outs are needed to minimize muscle artifacts and auditory-evoked potentials, while maximizing cortical signals [56, 57]. TMS-EEG data acquisition protocols that have been developed on high-density, 64-electrode EEG systems will need to be simplified to allow for robust PCI measurement using standard, 19-electrode clinical EEG systems [58].
Given that only one TMS-evoked potential is needed to assess a patient’s capacity for consciousness, another future goal is development of neuroimaging priors to guide TMS targeting (Figure 1A). Currently, comprehensive cortical mapping finds the optimal ‘entry point’ that generates the highest PCI value. Multiple cortical sites are tested, requiring approximately 200 stimulations at each site (~ 8 minutes per site). By targeting cortical regions with high levels of structural or functional connectivity, as identified by diffusion MRI and resting-state fMRI, respectively, the chances of obtaining optimal complexity measures are likely to rise, reducing acquisition time to that of other bedside diagnostic tests performed in the ICU that range from 30–60 minutes in duration.
Finally, there is an urgent need to validate a standardized analytic pipeline for TMS-EEG data that will provide clinicians and families with reliable brain complexity information in real-time. TMS-EEG has the potential to be deployed at the bedside for serial assessment of brain complexity, similar to the daily assessments of cerebral blood flow velocity performed with transcranial doppler ultrasound for patients with aneurysmal subarachnoid hemorrhage. Serial measurement of brain complexity is crucial for patients with DoC because of frequent fluctuations in their level of consciousness [59].
Safety Considerations
The safety of TMS-EEG is well-established, especially when TMS pulses are delivered at low frequency (<1 Hz), as they are for calculating brain complexity. Minor side effects, such as scalp discomfort or headache, have been reported [60]. These side effects can be mitigated by modifying the TMS stimulation target if muscle twitching is observed. A survey involving 174 labs and 318,560 TMS sessions showed that the risk of inducing seizures, the most serious potential side effect, is below 1 in 10,000 when TMS is used within the safety guidelines and in low-frequency mode, even in patients at high risk of seizures [9, 61]. Further studies are needed to determine whether the incidence of seizures in patients with acute severe brain injuries is similarly low [19, 53, 62–64].
The Clinical Trial Horizon
Prior studies have applied TMS-EEG in the subacute and chronic care settings [18, 19, 65]. Attention should now turn to optimizing TMS-EEG for use in the acute ICU setting and performing an international, multi-center trial to validate its diagnostic and prognostic utility. To achieve this goal, partnerships between the neuroscience and clinical communities will need to be strengthened, along with ties to engineers developing faster, more compact TMS-EEG devices. The design of such technologies will allow validation of standardized data acquisition and analysis procedures that yield reliable PCI measurements across sites. There is also a need for common data elements (CDEs) to facilitate standardized reporting of TMS-EEG results, a goal that is now being pursued by the Curing Coma Campaign [66]. Additional applications in future clinical trials include the use of TMS-EEG to predict individualized responses to therapies [67], and to measure subclinical therapeutic responses [68]. It may also be possible for TMS-EEG to predict a patient’s risk of delirium [69, 70], a common ICU complication.
Conclusions
In the decades-long search for a “consciousness-detector”, TMS-EEG provides performance characteristics that surpass those of other advanced neurotechnologies. International guidelines have begun to endorse the clinical application of TMS-EEG to patients with DoC. Yet logistical and methodological barriers currently prevent clinical implementation of TMS-EEG in the ICU. Translation of TMS-EEG to the ICU has the potential to provide clinicians and families with a reliable index of consciousness – one that could substantially impact decisions about the continuation of life-sustaining therapy. We advocate for multicenter trials to test the reliability of TMS-EEG measures of PCI in critically ill patients with severe brain injuries to meet these clinical and ethical imperatives.
Acknowledgments:
We thank Kimberly Main Knoper for contributing the artwork for Figure 1.
Funding:
This work was supported by the Tiny Blue Dot Foundation, NIH Director’s Office (DP2HD101400), NIH National Institute of Neurological Disorders and Stroke (R21NS109627, K23NS112473), NIH BRAIN Initiative (F32MH123001), Italian Ministry of Health (Ricerca Corrente 2022–2024 and GR 2016–02361494), Fondazione Regionale per la Ricerca Biomedica (Regione Lombardia: Project ERAPERMED2019–101, GA779282), EU Grant No. 945539 (Human Brain Project SGA3), the James S. McDonnell Foundation, and the American Academy of Neurology (Palatucci Advocacy Leadership Award).
Footnotes
Conflicts of Interest:
C.K. and G.T. are Board Members, and C.K., G.T., and M.M. have a financial interest in Intrinsic Powers Inc. G.T. holds patent rights along with the Wisconsin Alumni Research Foundation (WARF): patent No. US 8,457,731 B2, and patent applications WARF No. P220191, P220192.
Ethical Approval: New data were not acquired or analyzed for this manuscript, and therefore there was no need for informed consent or approval from an Institutional Review Board.
References
- 1.Giacino JT and Kalmar K, The vegetative and minimally conscious states: A comparison of clinical features and functional outcome. J Head Trauma Rehabil, 1997; 12: 36–51. [Google Scholar]
- 2.Roozenbeek B, et al. , Prediction of outcome after moderate and severe traumatic brain injury: external validation of the International Mission on Prognosis and Analysis of Clinical Trials (IMPACT) and Corticoid Randomisation After Significant Head injury (CRASH) prognostic models. Crit Care Med, 2012; 40: 1609–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Claassen J, et al. , Detection of Brain Activation in Unresponsive Patients with Acute Brain Injury. N Engl J Med, 2019; 380: 2497–2505. [DOI] [PubMed] [Google Scholar]
- 4.Egbebike J, et al. , Cognitive-motor dissociation and time to functional recovery in patients with acute brain injury in the USA: a prospective observational cohort study. Lancet Neurol, 2022; 21: 704–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koch C, et al. , Neural correlates of consciousness: progress and problems. Nat Rev Neurosci, 2016; 17: 307–21. [DOI] [PubMed] [Google Scholar]
- 6.Edlow BL, et al. , Early detection of consciousness in patients with acute severe traumatic brain injury. Brain, 2017; 140: 2399–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maas AIR, et al. , Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol, 2017; 16: 987–1048. [DOI] [PubMed] [Google Scholar]
- 8.Helbok R, et al. , The Curing Coma Campaign International Survey on Coma Epidemiology, Evaluation, and Therapy (COME TOGETHER). Neurocrit Care, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edlow BL, et al. , Recovery from disorders of consciousness: mechanisms, prognosis and emerging therapies. Nat Rev Neurol, 2021; 17: 135–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engemann DA, et al. , Robust EEG-based cross-site and cross-protocol classification of states of consciousness. Brain, 2018; 141: 3179–3192. [DOI] [PubMed] [Google Scholar]
- 11.Frohlich J, Toker D, and Monti MM, Consciousness among delta waves: a paradox? Brain, 2021; 144: 2257–2277. [DOI] [PubMed] [Google Scholar]
- 12.Cruse D, et al. , Bedside detection of awareness in the vegetative state: a cohort study. Lancet, 2011; 378: 2088–94. [DOI] [PubMed] [Google Scholar]
- 13.Tononi G, et al. , Integrated information theory: from consciousness to its physical substrate. Nat Rev Neurosci, 2016; 17: 450–61. [DOI] [PubMed] [Google Scholar]
- 14.Tononi G, Consciousness as integrated information: a provisional manifesto. Biol Bull, 2008; 215: 216–42. [DOI] [PubMed] [Google Scholar]
- 15.Tononi G and Sporns O, Measuring information integration. BMC Neurosci, 2003; 4: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mediano PAM, et al. , The strength of weak integrated information theory. Trends Cogn Sci, 2022; 26: 646–655. [DOI] [PubMed] [Google Scholar]
- 17.Sarasso S, et al. , Consciousness and complexity: a consilience of evidence. Neurosci Conscious, 2021; 7: 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casali AG, et al. , A theoretically based index of consciousness independent of sensory processing and behavior. Sci Transl Med, 2013; 5: 198ra105. [DOI] [PubMed] [Google Scholar]
- 19.Casarotto S, et al. , Stratification of unresponsive patients by an independently validated index of brain complexity. Ann Neurol, 2016; 80: 718–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koch C and Mackenzie A, How to Make a Consciousness Meter. Scientific American, 2017; 317: 28–33. [DOI] [PubMed] [Google Scholar]
- 21.Curley WH, et al. , Electrophysiological correlates of thalamocortical function in acute severe traumatic brain injury. Cortex, 2022; 152: 136–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Latronico N and Bolton CF, Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol, 2011; 10: 931–41. [DOI] [PubMed] [Google Scholar]
- 23.Bardin JC, et al. , Dissociations between behavioural and functional magnetic resonance imaging-based evaluations of cognitive function after brain injury. Brain, 2011; 134: 769–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young MJ, et al. , Toward uniform insurer coverage for functional MRI following severe brain injury. J Head Trauma Rehabil, 2023; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Comanducci A, et al. , Unconscious or unresponsiveness in akinetic mutism? Insights from a multimodal longitudinal exploration. Authorea, 2022; DOI: 10.22541/au.167243614.45733481/v1. [DOI] [PubMed] [Google Scholar]
- 26.Norton L, et al. , Functional Neuroimaging as an Assessment Tool in Critically Ill Patients. Ann Neurol, 2023; 93: 131–141. [DOI] [PubMed] [Google Scholar]
- 27.Teasdale G and Jennett B, Assessment of coma and impaired consciousness. A practical scale. Lancet, 1974; 2: 81–4. [DOI] [PubMed] [Google Scholar]
- 28.Wijdicks EF, et al. , Validation of a new coma scale: The FOUR score. Ann Neurol, 2005; 58: 585–93. [DOI] [PubMed] [Google Scholar]
- 29.Giacino JT, Kalmar K, and Whyte J, The JFK Coma Recovery Scale-Revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil, 2004; 85: 2020–9. [DOI] [PubMed] [Google Scholar]
- 30.Pincherle A, et al. , Motor behavior unmasks residual cognition in disorders of consciousness. Ann Neurol, 2019; 85: 443–447. [DOI] [PubMed] [Google Scholar]
- 31.Elmer J, et al. , Association of early withdrawal of life-sustaining therapy for perceived neurological prognosis with mortality after cardiac arrest. Resuscitation, 2016; 102: 127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turgeon AF, et al. , Mortality associated with withdrawal of life-sustaining therapy for patients with severe traumatic brain injury: a Canadian multicentre cohort study. CMAJ, 2011; 183: 1581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fins JJ, Rights come to mind: Brain injury, ethics, and the struggle for consciousness. 2015, New York, NY: Cambridge University Press. [Google Scholar]
- 34.Young MJ, et al. , The neuroethics of disorders of consciousness: a brief history of evolving ideas. Brain, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Young MJ and Edlow BL, The Quest for Covert Consciousness: Bringing Neuroethics to the Bedside. Neurology, 2021; 96: 893–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fins JJ and Bernat JL, Ethical, palliative, and policy considerations in disorders of consciousness. Neurology, 2018; 91: 471–475. [DOI] [PubMed] [Google Scholar]
- 37.Fischer D, et al. , Disorders of Consciousness Associated With COVID-19: A Prospective, Multimodal Study of Recovery and Brain Connectivity. Neurology, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fischer D, et al. , Neuroprognostication: a conceptual framework. Nat Rev Neurol, 2022; 18: 419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edlow BL and Fins JJ, Assessment of Covert Consciousness in the Intensive Care Unit: Clinical and Ethical Considerations. J Head Trauma Rehabil, 2018; 33: 424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peterson A, Aas S, and Wasserman D, What Justifies the Allocation of Health Care Resources to Patients with Disorders of Consciousness? AJOB Neurosci, 2021; 12: 127–139. [DOI] [PubMed] [Google Scholar]
- 41.Peterson A, et al. , Caregiver reactions to neuroimaging evidence of covert consciousness in patients with severe brain injury: a qualitative interview study. BMC Med Ethics, 2021; 22: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Young MJ, Bodien YG, and Edlow BL, Ethical Considerations in Clinical Trials for Disorders of Consciousness. Brain Sci, 2022; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Owen AM, et al. , Detecting awareness in the vegetative state. Science, 2006; 313: 1402. [DOI] [PubMed] [Google Scholar]
- 44.Giacino JT, et al. , Comprehensive systematic review update summary: Disorders of consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Neurology, 2018; 91: 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kondziella D, et al. , European Academy of Neurology guideline on the diagnosis of coma and other disorders of consciousness. Eur J Neurol, 2020; 27: 741–756. [DOI] [PubMed] [Google Scholar]
- 46.Comanducci A, et al. , Clinical and advanced neurophysiology in the prognostic and diagnostic evaluation of disorders of consciousness: review of an IFCN-endorsed expert group. Clin Neurophysiol, 2020; 131: 2736–2765. [DOI] [PubMed] [Google Scholar]
- 47.Scolding N, Owen AM, and Keown J, Prolonged disorders of consciousness: a critical evaluation of the new UK guidelines. Brain, 2021; 144: 1655–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Monti MM and Schnakers C, Flowchart for Implementing Advanced Imaging and Electrophysiology in Patients With Disorders of Consciousness: To fMRI or Not to fMRI? Neurology, 2022; 98: 452–459. [DOI] [PubMed] [Google Scholar]
- 49.Belardinelli P, et al. , Reproducibility in TMS-EEG studies: A call for data sharing, standard procedures and effective experimental control. Brain Stimul, 2019; 12: 787–790. [DOI] [PubMed] [Google Scholar]
- 50.Tremblay S, et al. , Clinical utility and prospective of TMS-EEG. Clin Neurophysiol, 2019; 130: 802–844. [DOI] [PubMed] [Google Scholar]
- 51.Julkunen P, Kimiskidis VK, and Belardinelli P, Bridging the gap: TMS-EEG from lab to clinic. J Neurosci Methods, 2022; 369: 109482. [DOI] [PubMed] [Google Scholar]
- 52.Lioumis P and Rosanova M, The role of neuronavigation in TMS-EEG studies: Current applications and future perspectives. J Neurosci Methods, 2022; 380: 109677. [DOI] [PubMed] [Google Scholar]
- 53.Rosanova M, et al. , Recovery of cortical effective connectivity and recovery of consciousness in vegetative patients. Brain, 2012; 135: 1308–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iglesias JE, et al. , Joint super-resolution and synthesis of 1 mm isotropic MP-RAGE volumes from clinical MRI exams with scans of different orientation, resolution and contrast. Neuroimage, 2021; 237: 118206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iglesias JE, et al. , SynthSR: A public AI tool to turn heterogeneous clinical brain scans into high-resolution T1-weighted images for 3D morphometry. Sci Adv, 2023; 9: eadd3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Casarotto S, et al. , The rt-TEP tool: real-time visualization of TMS-Evoked Potentials to maximize cortical activation and minimize artifacts. J Neurosci Methods, 2022; 370: 109486. [DOI] [PubMed] [Google Scholar]
- 57.Russo S, et al. , TAAC - TMS Adaptable Auditory Control: A universal tool to mask TMS clicks. J Neurosci Methods, 2022; 370: 109491. [DOI] [PubMed] [Google Scholar]
- 58.Comolatti R, et al. , A fast and general method to empirically estimate the complexity of brain responses to transcranial and intracranial stimulations. Brain Stimul, 2019; 12: 1280–1289. [DOI] [PubMed] [Google Scholar]
- 59.Wannez S, et al. , The repetition of behavioral assessments in diagnosis of disorders of consciousness. Ann Neurol, 2017; 81: 883–889. [DOI] [PubMed] [Google Scholar]
- 60.Rossi S, et al. , Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert Guidelines. Clin Neurophysiol, 2021; 132: 269–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lerner AJ, Wassermann EM, and Tamir DI, Seizures from transcranial magnetic stimulation 2012–2016: Results of a survey of active laboratories and clinics. Clin Neurophysiol, 2019; 130: 1409–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gosseries O, et al. , On the cerebral origin of EEG responses to TMS: insights from severe cortical lesions. Brain Stimul, 2015; 8: 142–9. [DOI] [PubMed] [Google Scholar]
- 63.Rosanova M, et al. , Sleep-like cortical OFF-periods disrupt causality and complexity in the brain of unresponsive wakefulness syndrome patients. Nat Commun, 2018; 9: 4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ragazzoni A, et al. , Vegetative versus minimally conscious states: a study using TMS-EEG, sensory and event-related potentials. PLoS One, 2013; 8: e57069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sinitsyn DO, et al. , Detecting the Potential for Consciousness in Unresponsive Patients Using the Perturbational Complexity Index. Brain Sci, 2020; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Provencio JJ, et al. , The Curing Coma Campaign: Framing Initial Scientific Challenges-Proceedings of the First Curing Coma Campaign Scientific Advisory Council Meeting. Neurocrit Care, 2020; 33: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Edlow BL, et al. , Personalized Connectome Mapping to Guide Targeted Therapy and Promote Recovery of Consciousness in the Intensive Care Unit. Neurocrit Care, 2020; 33: 364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scott G and Carhart-Harris RL, Psychedelics as a treatment for disorders of consciousness. Neurosci Conscious, 2019; 2019: niz003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ross JM, et al. , Neurophysiologic predictors of individual risk for post-operative delirium after elective surgery. J Am Geriatr Soc, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bai Y, et al. , Cortical reactivity to transcranial magnetic stimulation predicts risk of post-stroke delirium. Clin Neurophysiol, 2022. [DOI] [PubMed] [Google Scholar]


