Abstract
Background
Young women with breast cancer (BC) have an increased chance of carrying germline BRCA pathogenic variants (PVs). Limited data exist on the prognostic impact of tumor histology (i.e. ductal versus lobular) in hereditary breast cancer.
Methods
This multicenter retrospective cohort study included women aged ≤40 years with early-stage breast cancer diagnosed between January 2000 and December 2020 and known to carry germline PVs in BRCA1/2. Histology was locally assessed in each center. The Kaplan–Meier method and Cox regression analysis were used to assess disease-free survival and overall survival.
Results
Of 4628 patients included from 78 centers worldwide, 3969 (86%) had pure ductal, 135 (3%) pure lobular, and 524 (11%) other histologies. Compared with ductal tumors, lobular tumors were more often grade 1/2 (57.7% versus 22.1%), stage III (29.6% versus 18.5%), and luminal A-like (42.2% versus 12.2%). Lobular tumors were more often associated with BRCA2 PVs (71.1% BRCA2), while ductal tumors were more often associated with BRCA1 PVs (65.7% BRCA1). Patients with lobular tumors more often had mastectomy (68.9% versus 58.3%), and less often received chemotherapy (83.7% versus 92.9%). With a median follow-up of 7.8 years, no significant differences were observed in disease-free survival (adjusted hazard ratio 1.01, 95% confidence interval 0.74-1.37) or overall survival (hazard ratio 0.96, 95% confidence interval 0.62-1.50) between patients with ductal versus lobular tumors. No significant survival differences were observed according to specific BRCA gene, breast cancer subtype, or body mass index.
Conclusions
In this large global cohort of young BRCA carriers with breast cancer, the incidence of pure lobular histology was low and associated with higher disease stage at diagnosis, luminal-like disease and BRCA2 PVs. Histology did not appear to impact prognosis.
Key words: BRCA, breast cancer, histology, lobular
Highlights
-
•
We analyzed 4628 young BRCA carriers with early breast cancer.
-
•
The incidence of pure lobular histology was low (i.e. 3%).
-
•
Patients with lobular cancers had higher disease stage at diagnosis, more luminal-like disease, and more BRCA2 PVs.
-
•
Histology did not appear to impact prognosis.
Introduction
Breast cancers can be categorized into different histological subtypes based on cell morphology, growth, and architectural patterns.1 The most common histology accounting for ∼75%-80% of all invasive breast cancers is invasive carcinoma of no special type, commonly known as invasive ductal carcinoma.2 The remaining 20%-25% of breast cancers consist of special histologies, with lobular breast cancer being the most prevalent among them.2 Compared with ductal tumors, lobular cancers exhibit differences in clinical presentation,2 long-term outcomes,3 site of disease progression,4 histopathological characteristics,5 and biological features.6 Current international guidelines provide some specific indications for selected breast cancer of special histologies (e.g. adenoid cystic, secretory, medullary), but not specifically on lobular tumors, in part due to limited research focusing on histologic distinctions.7
In particular, the prognostic impact of different histologies in breast cancers arising in young patients with a germline BRCA pathogenic or likely pathogenic variant (PV) has not been previously extensively studied. Limited data exist on the prevalence of lobular tumors among young BRCA carriers,8,9 as well as on the clinicopathological characteristics and survival outcomes of this specific subset of patients.
The BRCA BCY Collaboration (NCT03673306) is the largest international cohort study including young women with breast cancer carrying germline BRCA PVs.10 With its large real-world cohort of young BRCA carriers with breast cancer, this study represents a unique opportunity to explore the impact of the different histologies on the distribution of clinicopathological characteristics and on patients’ clinical outcomes.
Methods
Study design and procedures
This international retrospective observational cohort study, conducted across 78 centers worldwide, included women aged ≤40 years who were diagnosed with invasive breast cancer between January 2000 and December 2020 and tested positive for germline BRCA1/2 PVs. Criteria for inclusion/exclusion in the study were previously detailed.10 Information collected included country of treatment, year and age at diagnosis, menopausal status, type of BRCA1/2 PV, primary tumor size, lymph node involvement at diagnosis, histology, grade, human epidermal growth factor receptor 2 (HER2) and hormone receptor status as well as administered treatments [type of surgery, type of chemotherapy (if administered), type of endocrine therapy (if administered)]. Histology, grade, HER2, and hormone receptor status were locally assessed and no central review was carried out.
Breast cancer subtypes were centrally defined following immunohistochemistry and/or in situ hybridization-based criteria (according to the current guidelines)11,12 as follows13: (i) luminal A-like, as estrogen receptor (ER)- and progesterone receptor (PgR)-positive, HER2-negative tumors of grade 1 or 2; (ii) luminal B-like, as either ER-negative/PgR-positive or ER-positive/PgR-negative, HER2-negative tumors, or ER- and/or PgR-positive, HER2-negative tumors of grade 3; (iii) HER2-positive as tumors with positive HER2 expression (regardless of other characteristics); (iv) triple-negative breast cancer (TNBC) as ER- and PgR-negative, HER2-negative tumors (any grade).
BRCA testing was locally carried out and re-classifications over time were eventually applied by each site as per internal guidelines.
The study was approved by the ethical review committee of the Institut Jules Bordet (Brussels, Belgium) as coordinating center. Whenever required, ethics approval was obtained in compliance with local regulations from independent ethical review committees or institutional review boards of participating centers.
Study objectives
The primary objective of this analysis was to investigate the impact of the different histologies of breast cancer on the clinical outcomes of young BRCA carriers (pure ductal versus pure lobular).
The impact of histologies on prognosis was investigated by comparing the following time-to-event endpoints, defined as per STEEP criteria14: disease-free survival (DFS), defined by the occurrence of one of the following invasive events: local recurrence, distant metastases, contralateral or ipsilateral breast tumor, second primary malignancy, or death from any cause; overall survival (OS), defined by the occurrence of death from any cause.
Secondary objectives included: describing baseline patient and tumor characteristics according to the different histologies; investigating the impact of the different histologies on the clinical outcomes of young BRCA carriers according to the type of specific BRCA gene (BRCA 1 versus BRCA 2), breast cancer subtype (luminal A-like, luminal B-like, HER2-positive, TNBC), and body mass index (BMI) (<25 versus ≥25).
Statistical analysis
Categorical and continuous variables of ductal versus lobular tumor were compared using the chi-square test and Wilcoxon rank-sum test as appropriate.
The Kaplan–Meier method was used to compute survival probabilities. The log-rank test was used to compare survival probabilities. Cox proportional hazards model was used to calculate unadjusted and adjusted hazard ratios (HR) with 95% confidence interval (CI). Adjustment in survival models was made for age, specific BRCA gene, year of diagnosis, tumor grade, tumor size, nodal status, tumor subtype, breast surgery, and use of chemotherapy. Cox models were used to assess the impact of different histologies on the clinical outcomes (i.e. DFS and OS) according to the type of specific BRCA gene (BRCA1 versus BRCA2), breast cancer subtype (luminal A-like, luminal B-like, HER2-positive, TNBC), and BMI (<25 versus ≥25). The presence of interaction between histology and type of specific BRCA gene (1 versus 2), breast cancer subtypes, or BMI were also assessed. For survival analysis, patients whose tumors had mixed histologies (i.e. mixed ductal and lobular) were excluded from the comparative analysis of ductal versus lobular tumors.
Results
Population characteristics and treatment received
A total of 4628 patients were included, of whom 3969 (86%) had pure ductal, 135 (3%) pure lobular, and 524 (11%) other histologies, respectively (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2024.103714). Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2024.103714 reports the baseline characteristics of patients with tumors of pure ductal, pure lobular or other histology.
Comparisons of baseline clinicopathological characteristics between patients with pure ductal and pure lobular tumors are shown in Table 1. Compared with ductal carcinoma, lobular tumors were more often of grade 1/2 (57.7% versus 22.1%, P < 0.001), of stage III (29.6% versus 18.5%, P = 0.002), and of luminal A-like subtype (42.2% versus 12.2%, P < 0.001). Lobular tumors were more often associated with BRCA2 PVs (71.1% had BRCA2 PVs), while ductal tumors were more often associated with BRCA1 PVs (65.7% had BRCA1 PVs). Patients with lobular tumors more often underwent mastectomy (68.9% versus 58.3%, P = 0.011), and less often chemotherapy (83.7% versus 92.9%, P < 0.001).
Table 1.
Baseline clinicopathological characteristics
| Pure ductal BC n (%) n = 3969 |
Pure lobular BC n (%) n = 135 |
P value | |
|---|---|---|---|
| Region | 0.002 | ||
| North America | 319 (8.0) | 11 (8.1) | |
| South-Center America | 130 (3.3) | 5 (3.7) | |
| Asia + Israel | 727 (18.3) | 7 (5.2) | |
| Oceania | 140 (3.5) | 4 (3.0) | |
| North Europe | 620 (15.6) | 28 (20.7) | |
| South Europe | 1793 (45.2) | 65 (48.1) | |
| East Europe | 240 (6.0) | 15 (11.1) | |
| Year of diagnosis | 0.053 | ||
| 2000-2004 | 498 (12.5) | 25 (18.5) | |
| 2005-2008 | 636 (16.0) | 28 (20.7) | |
| 2009-2012 | 867 (21.8) | 19 (14.1) | |
| 2013-2016 | 966 (24.3) | 31 (23.0) | |
| 2017-2020 | 1002 (25.2) | 32 (23.7) | |
| Age at diagnosis, median (IQR) years | 35 (31 to 37) | 36 (33 to 39) | 0.018 |
| Menopausal status at diagnosis | 0.068 | ||
| Premenopausal | 3774 (95.1) | 124 (91.8) | |
| Peri-menopausal | 13 (0.3) | 1 (0.7) | |
| Post-menopausal | 91 (2.3) | 7 (5.2) | |
| Missing | 91 (2.3) | 3 (2.2) | |
| BRCA status | <0.001 | ||
| BRCA1 | 2606 (65.7) | 38 (28.1) | |
| BRCA2 | 1335 (33.6) | 96 (71.1) | |
| BRCA1 and BRCA2 | 23 (0.6) | 1 (0.7) | |
| BRCA pathogenic variant (unknown if 1 or 2) | 5 (0.1) | 0 (0.0) | |
| Time from diagnosis to BRCA testing, median (IQR) months | 5.3 (0.9-25.3) | 8.1 (1.5-44.6) | 0.081 |
| Missing date of BRCA testing | 500 | 13 | |
| Body mass index | 0.741 | ||
| <18.5 | 207 (5.2) | 9 (6.7) | |
| 18.5-24.9 | 2132 (53.7) | 72 (53.3) | |
| 25.0-29.9 | 660 (16.6) | 26 (19.3) | |
| ≥30 | 350 (8.8) | 10 (7.4) | |
| Missing | 620 (15.6) | 18 (13.3) | |
| Stage at breast cancer diagnosis | 0.002 | ||
| I | 1053 (26.5) | 25 (18.5) | |
| II | 2092 (52.7) | 67 (49.6) | |
| III | 734 (18.5) | 40 (29.6) | |
| Missing | 90 (2.3) | 3 (2.2) | |
| Tumor grade | <0.001 | ||
| G1 | 53 (1.3) | 11 (8.1) | |
| G2 | 826 (20.8) | 67 (49.6) | |
| G3 | 2774 (69.9) | 40 (29.6) | |
| Missing | 316 (8.0) | 17 (12.6) | |
| Tumor size | 0.001 | ||
| T1 | 1545 (38.9) | 42 (31.1) | |
| T2 | 1764 (44.4) | 53 (39.3) | |
| T3-T4 | 532 (13.4) | 33 (24.4) | |
| Missing | 128 (3.2) | 7 (5.2) | |
| Nodal status | <0.001 | ||
| N0 | 2063 (52.0) | 48 (35.6) | |
| N1 | 1321 (33.3) | 59 (43.7) | |
| N2-N3 | 474 (11.9) | 25 (18.5) | |
| Missing | 111 (2.8) | 3 (2.2) | |
| Laterality | 0.104 | ||
| Left | 1905 (48.0) | 73 (54.1) | |
| Right | 1900 (47.9) | 61 (45.2) | |
| Bilateral | 152 (3.8) | 1 (0.7) | |
| Missing | 12 (0.3) | 0 (0.0) | |
| Breast surgery | 0.011 | ||
| Not done | 11 (0.3) | 1 (0.7) | |
| Breast conserving surgery | 1603 (40.4) | 37 (27.4) | |
| Mastectomy | 2314 (58.3) | 93 (68.9) | |
| Missing | 41 (1.0) | 4 (3.0) | |
| Tumor subtype | <0.001 | ||
| Luminal A | 484 (12.2) | 57 (42.2) | |
| Luminal B | 865 (21.8) | 33 (24.4) | |
| TNBC | 2049 (51.6) | 19 (14.1) | |
| HER2-positive | 282 (7.1) | 8 (5.9) | |
| Missing | 289 (7.3) | 18 (13.3) | |
| Use of radiotherapy | 0.576 | ||
| No | 1301 (32.8) | 42 (31.1) | |
| Yes | 2592 (65.3) | 93 (68.9) | |
| Missing | 76 (1.9) | 0 (0.0) | |
| Type of systemic treatment | 0.471 | ||
| Not done | 47 (1.2) | 1 (0.7) | |
| Neoadjuvant | 1713 (43.2) | 52 (38.5) | |
| Adjuvant | 2176 (54.8) | 81 (60.0) | |
| Missing | 33 (0.8) | 1 (0.7) | |
| Use of chemotherapy | <0.001 | ||
| No | 258 (6.5) | 21 (15.6) | |
| Yes | 3689 (92.9) | 113 (83.7) | |
| Missing | 22 (0.5) | 1 (0.7) | |
| Type of chemotherapy (for CT = yes) | 0.780 | ||
| Anthracycline- and taxane-based | 2607 (70.7) | 82 (72.6) | |
| Anthracycline-based | 697 (18.9) | 17 (15.0) | |
| Taxane-based | 155 (4.2) | 4 (3.5) | |
| Others | 113 (3.1) | 4 (3.5) | |
| Missing | 117 (3.2) | 6 (5.3) | |
| Use of endocrine therapy (for HR+) | 0.277 | ||
| No | 83 (4.8) | 3 (2.6) | |
| Yes | 1624 (93.9) | 111 (97.4) | |
| Missing | 23 (1.3) | 0 (0.0) | |
| Type of endocrine therapy (for HR+ and ET = yes) | 0.896 | ||
| Tamoxifen alone | 566 (34.8) | 37 (33.3) | |
| Tamoxifen + LHRHa | 465 (28.6) | 32 (28.8) | |
| LHRHa alone | 28 (1.7) | 1 (0.9) | |
| AI ± LHRHa | 277 (17.1) | 23 (20.7) | |
| Tamoxifen and AI (± LHRHa) | 250 (15.4) | 15 (13.5) | |
| Others | 23 (1.4) | 2 (1.8) | |
| Missing | 15 (0.9) | 1 (0.9) | |
| Use of anti-HER2 therapy (for HER2+) | 0.843 | ||
| No | 28 (9.9) | 1 (12.5) | |
| Yes | 243 (86.2) | 7 (87.5) | |
| Missing | 11 (3.9) | 0 (0.0) | |
| Bilateral risk-reducing mastectomy | 0.422 | ||
| No | 1651 (41.6) | 51 (37.8) | |
| Yes | 2257 (56.9) | 81 (60.0) | |
| Missing | 61 (1.5) | 3 (2.2) | |
| Bilateral risk-reducing salpingo-oophorectomy | 0.598 | ||
| No | 1849 (46.6) | 60 (44.4) | |
| Yes | 2045 (51.5) | 74 (54.8) | |
| Missing | 75 (1.9) | 1 (0.7) |
AI, aromatase inhibitor; BC, breast cancer; CT, chemotherapy; ET, endocrine therapy; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; IQR, interquartile range; LHRHa, luteinizing hormone-releasing hormone analog; TNBC, triple-negative breast cancer.
Survival analyses
Table 2 reports the incidence and type of disease-free and overall survival events in patients with pure ductal and pure lobular breast cancer. Lobular tumors had more locoregional recurrences than ductal tumors (12.6% versus 7.6%, P = 0.047). No significant differences were observed in terms of distant recurrences or secondary malignancies.
Table 2.
Type of disease-free events in young BRCA carriers with pure ductal versus pure lobular breast cancer
| Pure ductal BC n (%) n = 3969 |
Pure lobular BC n (%) n = 135 |
P value | |
|---|---|---|---|
| Follow-up (years), median (IQR) | 7.77 (4.47-12.62) | 8.25 (4.73-14.41) | |
| No events | 2529 (63.7) | 77 (57.0) | 0.122 |
| Locoregional recurrence | 301 (7.6) | 17 (12.6) | 0.047 |
| Distant recurrence | 446 (11.2) | 18 (13.3) | 0.410 |
| Second primary malignancy | 159 (4.0) | 2 (1.5) | 0.175 |
| Ovary | 91 (2.3) | 1 (0.7) | |
| Pancreas | 9 (0.2) | 0 (0.0) | |
| Cervix | 9 (0.2) | 0 (0.0) | |
| Colorectal | 11 (0.3) | 0 (0.0) | |
| Hematology | 8 (0.2) | 0 (0.0) | |
| Skin | 18 (0.5) | 1 (0.7) | |
| Thyroid | 9 (0.2) | 0 (0.0) | |
| Endometrial | 8 (0.2) | 0 (0.0) | |
| Upper gastrointestinal tract | 5 (0.1) | 0 (0.0) | |
| Other | 30 (0.8) | 0 (0.0) | |
| Second primary breast cancer | 496 (12.5) | 18 (13.3) | 0.791 |
| Death without any disease-free survival event | 38 (1.0) | 3 (2.2) | 0.151 |
BC, breast cancer; IQR, interquartile range.
With a median follow-up of 7.8 years (interquartile range 4.5-12.6 years), no significant differences were observed in survival outcomes between patients with ductal versus lobular tumors, neither in the unadjusted nor in the adjusted models (Table 3).
Table 3.
Multivariate analysis of disease-free and overall survival in young BRCA carriers with pure ductal versus pure lobular breast cancers
| Disease-free survival |
Overall survival |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Histological type | 0.536 | 0.987 | ||||
| Ductal | Ref. | — | Ref. | — | ||
| Lobular | 1.01 | 0.74-1.37 | 0.96 | 0.62-1.50 | ||
| Year of diagnosis | 0.717 | 0.047 | ||||
| 2000-2004 | Ref. | — | Ref. | — | ||
| 2005-2008 | 1.07 | 0.89-1.24 | 1.33 | 1.01-1.75 | ||
| 2009-2012 | 0.97 | 0.81-1.15 | 1.32 | 0.99-1.77 | ||
| 2013-2016 | 1.03 | 0.85-1.23 | 1.61 | 1.19-2.18 | ||
| 2017-2020 | 1.10 | 0.88-1.37 | 1.63 | 1.12-2.39 | ||
| Tumor grade | 0.066 | 0.115 | ||||
| G1 | Ref. | — | Ref. | — | ||
| G2 | 1.42 | 0.85-2.36 | 1.99 | 0.73-5.42 | ||
| G3 | 1.20 | 0.71-2.04 | 1.50 | 0.54-4.16 | ||
| Tumor size | <0.001 | <0.001 | ||||
| T1 | Ref. | — | Ref. | — | ||
| T2 | 1.14 | 1.01-1.29 | 1.53 | 1.24-1.89 | ||
| T3-T4 | 1.47 | 1.23-1.74 | 2.21 | 1.70-2.88 | ||
| Nodal status | <0.001 | <0.001 | ||||
| N0 | Ref. | — | Ref. | — | ||
| N1 | 1.30 | 1.14-1.47 | 1.78 | 1.44-2.18 | ||
| N2-N3 | 1.70 | 1.44-2.01 | 2.60 | 2.02-3.35 | ||
| Tumor subtype | 0.034 | 0.584 | ||||
| Luminal A | Ref. | — | Ref. | — | ||
| Luminal B | 1.02 | 0.80-1.31 | 1.19 | 0.81-1.75 | ||
| TNBC | 1.27 | 0.99-1.63 | 1.31 | 0.89-1.93 | ||
| HER2-positive | 1.04 | 0.79-1.36 | 1.18 | 0.76-1.82 | ||
| Breast surgery | <0.001 | 0.008 | ||||
| Breast conserving surgery | Ref. | — | Ref. | — | ||
| Not done | 3.02 | 1.53-5.95 | 4.26 | 1.92-9.46 | ||
| Mastectomy | 0.78 | 0.70-0.87 | 1.10 | 0.92-1.33 | ||
| Age at diagnosis, years | 0.055 | 0.997 | ||||
| ≤35 | Ref. | — | Ref. | — | ||
| >35 | 0.91 | 0.81-1.01 | 1.01 | 0.85-1.20 | ||
| Use of chemotherapy | <0.001 | 0.025 | ||||
| No | Ref. | — | Ref. | — | ||
| Yes | 0.60 | 0.48-0.75 | 0.57 | 0.39-0.84 | ||
| BRCA status | 0.827 | 0.532 | ||||
| BRCA1 | Ref. | — | Ref. | — | ||
| BRCA2 | 0.97 | 0.84-1.12 | 0.88 | 0.71-1.10 | ||
| BRCA1 and BRCA2 | 0.97 | 0.53-1.77 | 1.25 | 0.51-3.04 | ||
CI, confidence interval; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; TNBC, triple-negative breast cancer.
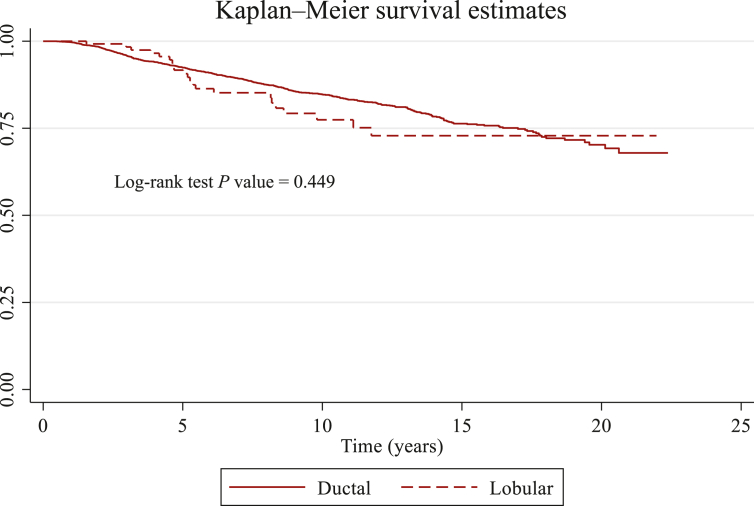
The 5-year DFS was 76% (95% CI 74% to 77%) in patients with ductal tumors, and 75% (95% CI 66% to 82%) in patients with lobular tumors (univariate Cox HR = 1.14, 95% CI 0.88-1.49; adjusted HR 1.01, 95% CI 0.74-1.37; Figure 1).
Figure 1.
Kaplan–Meier survival estimates of disease-free survival in young BRCA carriers with pure ductal versus pure lobular breast cancer.
The 5-year OS was 92% (95% CI 91% to 93%) in patients with ductal tumors, and 92% (95% CI 85% to 96%) in patients with lobular tumors (univariate Cox HR = 1.14, 95% CI 0.88-1.49; adjusted HR 0.96, 95% CI 0.62-1.50; Figure 2).
Figure 2.
Kaplan–Meier survival estimates of overall survival in young BRCA carriers with pure ductal versus pure lobular breast cancer.
No statistically significant interactions were observed between histology and type of specific BRCA gene [BRCA 1 versus 2; P for interaction (DFS) = 0.586, P for interaction (OS) = 0.246], breast cancer subtypes [P for interaction (DFS) = 0.886, P for interaction (OS) = 0.626], and BMI [P for interaction (DFS) = 0.250, P for interaction (OS) = 0.298].
No significant differences in survival outcomes were observed between patients with pure lobular versus pure ductal tumors according to type of specific BRCA gene (BRCA 1 versus 2) (Supplementary Tables S2 and S3 and Figures S2-S4, available at https://doi.org/10.1016/j.esmoop.2024.103714), breast cancer subtype (luminal-like, HER2-positive, TNBC) (Supplementary Tables S2 and S3 and Figures S5-S12, available at https://doi.org/10.1016/j.esmoop.2024.103714), and BMI (<25 versus ≥25) (Supplementary Tables S2 and S3 and Figures S13-S16, available at https://doi.org/10.1016/j.esmoop.2024.103714).
Discussion
In this large real-world cohort of young BRCA carriers with breast cancer, we assessed the impact of the different breast cancer histologies. Among the 4628 young patients included, we observed that the incidence of pure lobular histology was low, concerning only 3% of cases.1
Invasive lobular carcinoma represents ∼5%-15% of all breast cancer diagnoses, ranking as the second most common histology following invasive ductal carcinoma. Lobular tumors are recognized by their small, detached epithelial cells, with the majority being ER-positive and HER2-negative, and occurring more often in older women compared with ductal tumors.15,16
Our cohort included exclusively young women up to the age of 40 years at the time of diagnosis, and the majority of patients were carriers of a BRCA1 PV, that is most often associated with a TNBC phenotype. This might explain the low prevalence of lobular histology in our cohort, where more than one half of patients had a TNBC. Mavaddat et al.9 previously described a 4.5% prevalence of lobular tumors in their cohort of BRCA carriers, of which 2.2% were observed in BRCA1 and 8.3% in BRCA2 carriers; however, it should be considered that median age at diagnosis was 40 years in BRCA1 carriers and 43 years in BRCA2 carriers, respectively, which is older than the median age in our cohort.
Clinically, lobular tumors behave differently from ductal carcinoma, often appearing as multifocal or multicentric disease.17 Although information on multifocality was not available in our study, patients with lobular tumors more often had mastectomy compared with those with ductal tumors. Multifocality may have contributed to these surgical decisions. Of note, the detached cell nature and low cell density of lobular tumors contribute to the challenge in their clinical and radiological detection.18 This may underlie the relatively higher disease stage at diagnosis observed in our lobular cohort and the higher proportion of tumors with large tumor size (T3-T4) compared with the ductal carcinomas.
Additionally, lobular tumors display a distinct metastatic pattern involving the peritoneum, ovaries, gastrointestinal tract, leptomeninges, alongside common bone lesions.19 In our cohort, patients with lobular tumors experienced more locoregional recurrences compared with those with ductal tumors, with no significant differences in the rate of distant recurrences. It should be noted, however, that only the first recurrence event was analyzed, with information not available concerning possible distant recurrences after a locoregional recurrence.
From a therapeutic perspective, early-stage lobular breast cancers exhibit lower responsiveness to (neo)adjuvant chemotherapy than ductal cancers,20,21 and some studies suggested greater benefit from aromatase inhibitors than tamoxifen as adjuvant endocrine therapy.22 The latter consideration was recently questioned by the results of a large individual patient meta-analysis showing no evidence of differential efficacy for aromatase inhibitors over tamoxifen in lobular versus ductal carcinomas, and thus suggesting histology should not be considered as a predictive marker for differential endocrine treatment benefit.23 In our cohort, patients with lobular tumors received less chemotherapy than those with ductal tumors. We observed no significant differences on the type of endocrine therapy administered to patients with pure ductal versus pure lobular hormone receptor-positive tumors.
In our analysis, no significant differences were observed either in DFS or in OS between patients with ductal and lobular tumors, suggesting that in young BRCA carriers the histology does not appear to impact on prognosis. This is in contrast with some retrospective studies24,25 exploring prognosis of lobular tumors regardless of BRCA status. In a French retrospective cohort study of patients with metastatic breast cancer, lobular histology was identified as an independent adverse prognostic factor.25 Of note, the different settings (early versus metastatic) hamper a direct comparison between studies. In a Korean cohort study including more than 225 000 premenopausal patients, the breast cancer-specific survival of patients with stage I-III lobular breast cancer was significantly lower than that of patients with ductal tumors within the first 10 years after diagnosis.24 The presence of a BRCA germline mutation was not considered in this study. Overall, our data need to be carefully evaluated considering the small number of patients with lobular breast cancer (only 3%).
Besides their clinicopathological characterization, various genomic initiatives have attempted to characterize the molecular landscape of lobular breast cancer.26,27 PVs in ATM, BRCA2, CDH1, CHEK2, and PALB2 are associated with an increased risk of lobular breast cancer, while BRCA1 PVs are not.26 Mutations in CDH1, responsible for E-cadherin, the cell adhesion molecule, are nearly pathognomonic genomic events in lobular tumors, reported in up to 90% of cases.27, 28, 29 Thus, consistent evidence supports the fact that lobular breast cancer is a distinct clinical and biological entity. Nonetheless, with few exceptions,30 these patients typically undergo the same treatments and participate in the same clinical trials as those with ductal breast cancers.31 Moreover, many trials do not specifically report outcomes based on histology, complicating the extrapolation of data for patients with lobular tumors. Lobular breast cancers are often underrepresented in trials due to their diffuse growth pattern and lack of measurable disease.32 Consequently, addressing the numerous uncertainties and unmet needs in lobular breast cancer management requires tailored clinical studies for this specific patient subgroup.
Our study has some limitations that should be acknowledged, most of which are related to its retrospective nature. All information was extracted from medical records, and some potentially relevant variables (e.g. ethnicity, gene expression signature data) were not collected. Histology was locally assessed, and no central pathology review was carried out. Additionally, data were collected from multiple centers worldwide, with different health care systems and different drug availabilities. Patients were diagnosed over a period of 20 years, during which the treatment of early breast cancer has improved, particularly for patients with hormone receptor-positive disease, who represent the majority of patients with lobular tumors. Patients included toward the end of the study period had less observation time to evaluate outcomes and recurrences; this limitation is particularly important for the interpretation of survival data, especially patients with hormone receptor-positive disease. Finally, this study focused on young BRCA carriers, so our results are not necessarily generalizable to BRCA carriers of all ages.
Conclusions
In this large cohort of young women with breast cancer and known germline BRCA1/2 PVs, the incidence of pure lobular histology was low (i.e. 3%). Patients with lobular cancers had higher disease stage at diagnosis, more luminal-like disease and more BRCA2 PVs. Histology did not appear to impact prognosis. Prospective clinical trials that are dedicated to lobular breast cancer could further elucidate best practices related to the treatment of this distinct biologic entity.
Acknowledgements
The abstract of the present work was accepted for presentation at the ESMO Breast Cancer Annual Meeting 2024 and was awarded with an ESMO Merit Travel Grant Award.
EA acknowledges Dr Daphné t’Kint de Roodenbeke and Dr Masa Auprih, Institut Jules Bordet, Brussels (Belgium) for their contributions. JB acknowledges Prof. Rajeev Sarin and Cancer Genetic Clinic, Tata Memorial Centre, Mumbai for their contributions. RF acknowledges the Breast Unit and Medical Oncology Unit, Fondazione IRCCS San Gerardo, Monza (Italy) for their contribution.
Funding
This work was supported by the Italian Association for Cancer Research [AIRC grant number MFAG 2020 ID 24698 to the BRCA BCY Collaboration (NCT03673306)]. The European Society for Medical Oncology (ESMO) for a translational research fellowship at the Institut Jules Bordet in Brussels, Belgium, at the time this study was initiated [to ML] (no grant number). The Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea [grant number HC20C0135 to HJK]. Data collection for most Australian participants was through the kConFab Follow-Up Study with support from Cancer Australia and the National Breast Cancer Foundation [grant number PdCCRS 1100868], Cancer Australia [grant number 809195], the Australian National Breast Cancer Foundation (IF 17), the Australian National Health and Medical Research Council [grant numbers 454508, 288704, and 145684], the United States National Institutes of Health [grant number 1RO1CA159868], the Queensland Cancer Fund, the Cancer Councils of New South Wales, Victoria, Tasmania, and South Australia, and the Cancer Foundation of Western Australia. The Breast Cancer Research Foundation and Susan G. Komen [to AHP] (no grant number).
The study supporters had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Disclosure
EA: honoraria and/or advisory boards from: Eli Lilly, Sandoz, AstraZeneca. Research grant to my Institution from Gilead. travel grants from: Novartis, Roche, Eli Lilly, Genetic, Istituto Gentili, Daiichi Sankyo, AstraZeneca (all outside the submitted work). RBM: support for attending medical conferences from: Pfizer; speakers fee from Novartis, AstraZeneca (all outside the submitted work). SL: reports grants paid to the institute from AstraZeneca, Eurocept Plaza, Roche, Genentech, Gilead Sciences, GlaxoSmithKline (GSK), Novartis, and Agendia outside the submitted work; consulting fees from AstraZeneca paid to the institute; educational fees from Daiichi Sankyo paid to the institute; other financial support for attending meetings from Daiichi Sankyo; non-financial support from Genentech (drug), Roche (drug), Gilead Sciences (drug), Novartis (drug), Agendia (gene expression tests), and AstraZeneca (drug). SCL has a patent (PCT/EP2022/73958) pending on a method for assessing homologous recombination deficiency in ovarian cancer cells. KP: honoraria for consultations/lectures/training/clinical trials and payment of conferences fees from AstraZeneca, Gilead, Roche, Novartis, Eli Lilly, Pfizer, Merck Sharp & Dohme (MSD), Teva, Egis, and Vipharm (all outside the submitted work). FD: support for attending medical conferences from: Novartis, Gilead; speakers fee from Novartis. GNM: support to attend medical conference from AstraZeneca. LC: speaker honoraria from AstraZeneca, Pfizer, Novartis, Gilead, MSD; advisory role for AstraZeneca, Pfizer, MSD, Amgen, Roche. RDR: AstraZeneca, MSD, GSK, Janssen, Bayer, Orion Pharma. CRJ: honoraria (paid to my institution) from Theramex, advisory board (paid to my institution) from Theramex, Roche, Gedeon Richter, speakers bureau (paid to my institution) from Bristol Myers Squibb, Organon, Novartis, research funding (paid to my institution) from Gedeon Richter and Bayer Healthcare. WC: honoraria from Merck, Pfizer, and Eisai. CV: consultancy or role in advisory board: Eli Lilly, Novartis, Pfizer, Daiichi Sankyo, Menarini. Honoraria as a speaker: Novartis, Eli Lilly, Istituto Gentili, MSD, Accademia di Medicina. Research grants (to the institution): Roche. LP: speaker/advisor/investigator: MSD, BMS, Pfizer, AstraZeneca, Roche, Merck, Novartis, Lilly, Takeda, Helsinn, Astellas, Janssen, Sanofi, Sandoz, Actavis, Amgen, Archigen, Amicus, Taiho, Infinity, Bioclin, G1 Therapeutics, MEI Pharma, Immunocore/Medison, NAPO Pharmaceuticals, Oktal, PharmaSwiss, AbbVie, Medica Linea, MAK Pharma, Agendia, Recordati, Incyte. FP: honoraria for advisory boards, activities as a speaker, travel grants, research grants: Amgen, AstraZeneca, Daichii Sankyo, Celgene, Eisai, Eli Lilly, Exact Sciences, Gilead, Ipsen, Menarini, MSD, Novartis, Pierre Fabre, Pfizer, Roche, Seagen, Takeda, Viatris. Research funding: AstraZeneca, Eisai, Roche. EdA: Financial: Honoraria and/or advisory board from Roche/GNE, Novartis, SeaGen, Zodiac, Libbs, Pierre Fabre, Lilly, AstraZeneca, MSD, Gilead Sciences; travel grants from Roche/GNE and AstraZeneca; Research grant to my institution from Roche/GNE, AstraZeneca, and GSK/Novartis, Gilead Sciences; non-financial: ESMO director of Membership 2023-2025; BSMO President 2023-2026. EB: research funding (to the institution) from Gilead, speakers fee from Eli Lilly. ML: advisory role for Roche, Lilly, Novartis, AstraZeneca, Pfizer, Seagen, Gilead, MSD, Menarini, Daiichi Sankyo, and Exact Sciences; receiving speaker honoraria from Roche, Lilly, Novartis, Pfizer, Sandoz, Libbs, Daiichi Sankyo, Takeda, Knight, Ipsen, Gilead, and AstraZeneca; receiving travel grants from Gilead, Roche and Daiichi Sankyo; receiving research funding (to his institution) from Gilead; and having non-financial interests as the chair of the European Society for Medical Oncology (ESMO) Young Oncologists Committee (YOC) and as a member of the national council of the Italian Association of Medical Oncology. All other authors have declared no conflicts of interest.
Data sharing
Data are available upon reasonable request and submission of a research project proposal to the corresponding author, and after review and approval of the proposal by the BRCA BCY Collaboration.
Supplementary data
References
- 1.Tan P.H., Ellis I., Allison K., et al. The 2019 World Health Organization classification of tumours of the breast. Histopathology. 2020;77:181–185. doi: 10.1111/his.14091. [DOI] [PubMed] [Google Scholar]
- 2.Li C.I., Uribe D.J., Daling J.R. Clinical characteristics of different histologic types of breast cancer. Br J Cancer. 2005;93:1046–1052. doi: 10.1038/sj.bjc.6602787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adachi Y., Ishiguro J., Kotani H., et al. Comparison of clinical outcomes between luminal invasive ductal carcinoma and luminal invasive lobular carcinoma. BMC Cancer. 2016;16:248. doi: 10.1186/s12885-016-2275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathew A., Rajagopal P.S., Villgran V., et al. Distinct pattern of metastases in patients with invasive lobular carcinoma of the breast. Geburtshilfe Frauenheilkd. 2017;77:660–666. doi: 10.1055/s-0043-109374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Schepper M. Proceedings of the San Antonio Breast Cancer Symposium, December 7-10, 2021, Gonzalez Convention Center, San Antonio, Texas. American Association for Cancer Research; Philadelphia, PA: 2021. Results of a worldwide survey on the currently used histopathological diagnostic criteria for invasive lobular breast cancer (ILC) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciriello G., Gatza M.L., Beck A.H., et al. Comprehensive molecular portraits of invasive lobular breast cancer. Cell. 2015;163:506–519. doi: 10.1016/j.cell.2015.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loibl S., André F., Bachelot T., et al. Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2024;35:159–182. doi: 10.1016/j.annonc.2023.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Ditchi Y., Broudin C., El Dakdouki Y., et al. Low risk of invasive lobular carcinoma of the breast in carriers of BRCA1 (hereditary breast and ovarian cancer) and TP53 (Li-Fraumeni syndrome) germline mutations. Breast J. 2019;25:16–19. doi: 10.1111/tbj.13154. [DOI] [PubMed] [Google Scholar]
- 9.Mavaddat N., Barrowdale D., Andrulis I.L., et al. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the consortium of investigators of modifiers of BRCA1/2 (CIMBA) Cancer Epidemiol Biomarkers Prev. 2012;21:134–147. doi: 10.1158/1055-9965.EPI-11-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambertini M., Blondeaux E., Agostinetto E., et al. Pregnancy after breast cancer in young BRCA carriers: an international hospital-based cohort study. JAMA. 2024;331:49–59. doi: 10.1001/jama.2023.25463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolff A.C., Hammond M.E.H., Allison K.H., et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline focused update. Arch Pathol Lab Med. 2018;142:1364–1382. doi: 10.5858/arpa.2018-0902-SA. [DOI] [PubMed] [Google Scholar]
- 12.Allison K.H., Hammond M.E.H., Dowsett M., et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP Guideline update. J Clin Oncol. 2020;38:1346–1366. doi: 10.1200/JCO.19.02309. [DOI] [PubMed] [Google Scholar]
- 13.Partridge A.H., Hughes M.E., Warner E.T., et al. Subtype-dependent relationship between young age at diagnosis and breast cancer survival. J Clin Oncol. 2016;34:3308–3314. doi: 10.1200/JCO.2015.65.8013. [DOI] [PubMed] [Google Scholar]
- 14.Tolaney S.M., Garrett-Mayer E., White J., et al. Updated Standardized Definitions for Efficacy End Points (STEEP) in adjuvant breast cancer clinical trials: STEEP Version 2.0. J Clin Oncol. 2021;39:2720–2731. doi: 10.1200/JCO.20.03613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christgen M., Steinemann D., Kühnle E., et al. Lobular breast cancer: clinical, molecular and morphological characteristics. Pathol Res Pract. 2016;212:583–597. doi: 10.1016/j.prp.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Pestalozzi B.C., Zahrieh D., Mallon E., et al. Distinct clinical and prognostic features of infiltrating lobular carcinoma of the breast: combined results of 15 International Breast Cancer Study Group clinical trials. J Clin Oncol. 2008;26:3006–3014. doi: 10.1200/JCO.2007.14.9336. [DOI] [PubMed] [Google Scholar]
- 17.Iorfida M., Maiorano E., Orvieto E., et al. Invasive lobular breast cancer: subtypes and outcome. Breast Cancer Res Treat. 2012;133:713–723. doi: 10.1007/s10549-012-2002-z. [DOI] [PubMed] [Google Scholar]
- 18.Johnson K., Sarma D., Hwang E.S. Lobular breast cancer series: imaging. Breast Cancer Res. 2015;17:94. doi: 10.1186/s13058-015-0605-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guiu S., Wolfer A., Jacot W., et al. Invasive lobular breast cancer and its variants: how special are they for systemic therapy decisions? Crit Rev Oncol Hematol. 2014;92:235–257. doi: 10.1016/j.critrevonc.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Cocquyt V.F., Blondeel P.N., Depypere H.T., et al. Different responses to preoperative chemotherapy for invasive lobular and invasive ductal breast carcinoma. Eur J Surg Oncol. 2003;29:361–367. doi: 10.1053/ejso.2002.1404. [DOI] [PubMed] [Google Scholar]
- 21.Van Baelen K., Geukens T., Maetens M., et al. Current and future diagnostic and treatment strategies for patients with invasive lobular breast cancer. Ann Oncol. 2022;33:769–785. doi: 10.1016/j.annonc.2022.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Metzger Filho O., Giobbie-Hurder A., Mallon E., et al. Relative effectiveness of letrozole compared with tamoxifen for patients with lobular carcinoma in the BIG 1-98 trial. J Clin Oncol. 2015;33:2772–2779. doi: 10.1200/JCO.2015.60.8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hills R.K., Oesterreich S., Metzger O., et al. Abstract PD14-08: Effectiveness of aromatase inhibitors versus tamoxifen in lobular compared to ductal carcinoma: individual patient data meta-analysis of 9328 women with central histopathology, and 7654 women with e-Cadherin status. Cancer Res. 2002;82 PD14-08. [Google Scholar]
- 24.Yoon T.I., Jeong J., Lee S., et al. Survival outcomes in premenopausal patients with invasive lobular carcinoma. JAMA Netw Open. 2023;6 doi: 10.1001/jamanetworkopen.2023.42270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dalenc F., Lusque A., De La Motte Rouge T., et al. Impact of lobular versus ductal histology on overall survival in metastatic breast cancer: a French retrospective multicentre cohort study. Eur J Cancer. 2022;164:70–79. doi: 10.1016/j.ejca.2021.12.031. [DOI] [PubMed] [Google Scholar]
- 26.Yadav S., Hu C., Nathanson K.L., et al. Germline pathogenic variants in cancer predisposition genes among women with invasive lobular carcinoma of the breast. J Clin Oncol. 2021;39:3918–3926. doi: 10.1200/JCO.21.00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desmedt C., Zoppoli G., Gundem G., et al. Genomic characterization of primary invasive lobular breast cancer. J Clin Oncol. 2016;34:1872–1881. doi: 10.1200/JCO.2015.64.0334. [DOI] [PubMed] [Google Scholar]
- 28.Morrogh M., Andrade V.P., Giri D., et al. Cadherin-catenin complex dissociation in lobular neoplasia of the breast. Breast Cancer Res Treat. 2012;132:641–652. doi: 10.1007/s10549-011-1860-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dossus L., Benusiglio P.R. Lobular breast cancer: incidence and genetic and non-genetic risk factors. Breast Cancer Res. 2015;17:37. doi: 10.1186/s13058-015-0546-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agostinetto E., Nader-Marta G., Paesmans M., et al. ROSALINE: a phase II, neoadjuvant study targeting ROS1 in combination with endocrine therapy in invasive lobular carcinoma of the breast. Future Oncol. 2022;18:2383–2392. doi: 10.2217/fon-2022-0358. [DOI] [PubMed] [Google Scholar]
- 31.Trapani D., Curigliano G. How to treat lobular cancer in the adjuvant setting? Curr Opin Oncol. 2020;32:561–567. doi: 10.1097/CCO.0000000000000674. [DOI] [PubMed] [Google Scholar]
- 32.Abel M.K., Melisko M.E., Rugo H.S., et al. Decreased enrollment of patients with advanced lobular breast cancer compared to ductal breast cancer in interventional clinical trials. J Clin Oncol. 2021;39:1092. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.