Abstract
Rabies virus (RABV) is a neurotropic virus exclusively infecting neurons in the central nervous system. RABV encodes five proteins. Among them, the viral glycoprotein (RVG) plays a key role in viral entry into neurons and rabies pathogenesis. It was shown that the nature of the C-terminus of the RABV G protein, which possesses a PDZ-binding motif (PBM), modulates the virulence of the RABV strain. The neuronal protein partners recruited by this PBM may alter host cell function. This study was conducted to investigate the effect of RVG on synaptic function in the hippocampal dentate gyrus (DG) of rat. Two μl (108 T.U./ml) of the lentiviral vector containing RVG gene was injected into the DG of rat hippocampus. After 2 weeks, the rat’s brain was cross-sectioned and RVG-expressing cells were detected by fluorescent microscopy. Hippocampal synaptic activity of the infected rats was then examined by recording the local field potentials from DG after stimulation of the perforant pathway. Short-term synaptic plasticity was also assessed by double pulse stimulation. Expression of RVG in DG increased long-term potentiation population spikes (LTP-PS), whereas no facilitation of LTP-PS was found in neurons expressing δRVG (deleted PBM). Furthermore, RVG and δRVG strengthened paired-pulse facilitation. Heterosynaptic long-term depression (LTD) in the DG was significantly blocked in RVG-expressing group compared to the control group. This blockade was dependent to PBM motif as rats expressing δRVG in the DG-expressed LTD comparable to the RVG group. Our data demonstrate that RVG expression facilitates both short- and long-term synaptic plasticity in the DG indicating that it may involve both pre- and postsynaptic mechanisms to alter synaptic function. Further studies are needed to elucidate the underlying mechanisms.
Keywords: Long-term potentiation, Dentate gyrus, PDZ-binding motif, Plasticity, Rabies
Introduction
Rabies is still claiming 50,000 lives per year (Jackson 2010). The etiological agent, rabies virus (RABV), is a neurotropic virus affecting the central nervous system (CNS) of mammalian species. In spite of drastic behavioral changes, mild pathological alterations are usually seen in the rabies-infected brain. This phenomenon makes it difficult to understand the underlying mechanism of extreme behavioral amendments induced by rabies. The poor understanding of the pathogenic mechanisms of RABV may contribute to the failure of new treatment in infected patients. RABV encodes five structural proteins; glycoprotein, phosphoprotein, nucleoprotein, matrix protein, and RNA-dependent RNA polymerase (Tordo and Kouknetzoff 1993). RABV glycoprotein (RVG) is a trimeric type I trans-membrane protein and surface-exposed viral coat protein with an important role in the rabies pathogenesis by enabling the virus to bind to the receptors at the site of inoculation, to enter into neurons, and to fuse with the endosomal membrane. RVG is also involved in the viral gene transcription/translation and retrograde transport of the virus to upper neuronal cells (Cherian et al. 2015). Maintenance of the viability of RABV-infected cells is vital for the viral cycle and pathogenesis, yet the molecular mechanisms largely uncharacterized. There are some evidences indicating that incorporation of RVG into the membrane of target cells ascertains the fate of neuronal survival or death (Caillet-Saguy et al. 2015; Artola and Singer 1993).
The death of rabid patients with minor histological sign in their brains highlights the hypothesis that RABV may disrupt the function of nerve cells rather than destroy neurons (Bouzamondo et al. 1993; Tsiang 1993). Preservation of infected neurons is vital for viral dissemination (Lafon 2011). However, hijacking neurons through multiple signaling pathways (Azimzadeh Jamalkandi et al. 2016) may be reflected by aberrant synaptic transmission (Fu and Jackson 2005) or ion channels dysfunctions (Iwata et al. 1999) leading to rabies-associated clinical manifestations and ultimately death. Various RABV strains differently affect the homeostasis of infected neurons. Pathogenic or virulent RABV strains survive the host cells, maintain the cell integrity, and therefore guarantee viral propagation (Lafon 2011; Artola and Singer 1993). On the other hand, attenuated strains cause neuronal apoptosis, which precludes viral dissemination (Prehaud et al. 2003). It was shown that the difference in the cytoplasmic domain of RVG causes this dual action. The RVG cytoplasmic domain possesses a PDZ-binding motif (PBM). PBM is a protein–protein interaction domain typically recognized by proteins containing PDZ domains and plays a critical role in neuronal homeostasis. RABV may manipulate signaling pathway(s) involved in neuronal physiology through interaction with the PDZ domain of the neuronal microtubule-associated serine/threonine kinase 2 (MAST2). Meanwhile, it is reported that the extracellular domain of RVG can also inhibit neurotransmitter receptors thereby change the function of neurons (Lee and Zheng 2010). Yet, data upon the effect of RABV on the function of ion channels and synaptic transmission are limited. RABV has been shown to reduce voltage-dependent sodium and inward rectifier potassium channel currents in infected rodent neural cell lines (Iwata et al. 1999, 2000). It has been reported that release of neurotransmitters such as serotonin, dopamine, and norepinephrine is increased in the hippocampal region of the RABV-infected mice at the early stage of the disease (Fu and Jackson 2005). Infecting different cerebral regions, RABV elicits diverse behavioral changes from aggression to hallucination. Given that behavior is a reflection of changes in neuronal/synaptic activity in a specific neural circuit, exploring synaptic response to RABV or RVG will help to further reveal pathogenicity of rabies.
Hippocampus possesses a unique structure, which makes it ideal to study the synaptic function. Long-term potentiation (LTP) is one of the main cellular mechanisms of the hippocampus in dealing with new harmful or pleasant experiences. LTP is an experimental model of synaptic plasticity and an activity-dependent long-term strengthening of excitatory synaptic transmission following applying a transient train of high-frequency electrical stimulation in various cerebral regions including hippocampus (Bliss and Collingridge 1993; Fourcaudot et al. 2009). Another form of synaptic plasticity is long-term depression (LTD). LTD is an activity-dependent reduction in the efficacy of neuronal synapses lasting after a while following a patterned stimulus. As the opposing process to LTP, LTD is one of several processes that serves to selectively weaken specific synapses in order to make constructive use of synaptic strengthening caused by LTP. This is necessary because, if allowed to continue increasing in strength, synapses would ultimately reach a ceiling level of efficiency, which would inhibit the encoding of new information.
Thus, the present study aimed to examine the function of rat hippocampal synapses following infection with RVG.
Materials and Methods
Lentiviral Vector Production
The lentivectors were prepared according to the previously established method (Farzaneh et al. 2018). Briefly, HEK 293T cells (from Cell Bank of Pasteur Institute) were grown in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% FBS and 100 units/ml penicillin/streptomycin at 37 °C in a humidified 5% CO2 environment. Plasmids containing full-length RVG of challenge virus standard CVS (G-CVS) or δRVG, in which four amino acids in the PBM site are deleted (Fig. 1) (Khan et al. 2019), were kindly provided by Christophe Prehaud (Neuroimmunologie Virale, Institute Pasteur, Paris, France) and subcloned in Plenti7.3 which contains eGFP (Invitrogen, France). The HEK 293T cells were transfected with transfer (p-Lenti-G-CVS), envelope (G protein of VSV), and packaging (pMDLg, pRRE and pRSV/Rev) plasmids using traditional calcium phosphate method. Forty-eight hours after transfection, the medium was collected and filtered using a 0.45-µm filter (Macherey‐Nagel, Germany). The medium was then centrifuged at 50,000 rpm for 2 h at 4 °C and the viral pellet was suspended in PBS‐BSA 1% solution and stored at − 80 °C until use.
Fig. 1.
Structures of the RVG and δRVG
Determination of Viral Titration
Lentivirus titer was determined using fluorescent microscopy. Briefly, 1 × 104 HEK 293T cells/well were seeded in a 96-well plate. Twenty h later, they were infected by gradient dilution of the 10 µl lentiviral particles (1:10, 1:100, 1:1000, 1:104, 1:105 and 1:106) in 100 µl final volume of culture medium. Forty-eight hours later, fluorescent-positive cells were counted using fluorescent microscopy in triplicates and the number of positive cells was determined. Then, the lentivirus titer was estimated using the following formulation: Viral titer (T.U./ml) = number of fluorescent-positive cells × 10 × dilution.
Animals
Adult male Wistar rats (220–250 g, Pasteur Institute, n = 93) were used. They were housed individually in pairs in Plexiglas cages with free access to food and water in a 12-h light/12-h dark cycle. All efforts were made to use minimal number of rats and also diminish animal suffering during the study. Experiments were carried out in accordance with the Review Board and Ethics Committee of Pasteur Institute (Authorization code 93-0201-785, 22 April 2014) and conform to the European Communities Council Directive 2010-63-EU.
Stereotaxic Microinjection
Rats were anesthetized with intraperitoneal (i.p.) injection of ketamine (100 mg/kg) and xylazine (10 mg/kg). Then, 2 μl (108 T.U./ml) of RVG, or δRVG lentivirus, or phosphate buffer solution (PBS as control) was injected by stereotaxic surgery into the dentate gyrus (DG; AP = − 3.8, ML = 2.4, DV = − 3.5) using a Hamilton microsyringe.
Histology
Two weeks after injection of the lentivector, brain of the rats (three rats in each group) was dissected out for histological examinations. The animals were euthanized by CO2 asphyxiation and perfused transcardially with 4% paraformaldehyde in 0.1 M PBS. The brains were harvested, kept in 10% paraformaldehyde in 0.1 M PBS, and processed for embedding in paraffin blocks. The brains were then cut horizontally to 8 µm thickness. The sections were deparaffinized, rehydrated in a descending alcohol series, and stained with 4′,6-diamidino-2-phenylindole (DAPI, 100 ng/m in Tris-buffered saline, Sigma). The sections cover-slipped with 90% glycerol mounting buffer and visualized in the dark place with fluorescent microscope (Nikon, Japan) equipped with specific filter cube for FITC fluorescence channels and connected to a digital camera. Digital photographs were taken using 10 × objective lenses.
Electrophysiological Recordings
Two weeks after lentivector injection into DG, animals were anaesthetized by urethane (1.5 g/kg) and fixed in a stereotaxic apparatus. A bipolar-stimulating electrode, which was a twisted-pair stainless steel wires (A-M Systems, USA), was placed at the perforant path (AP: 8.1, ML: 4.3, DV: 3.3), and an analogous electrode was placed at DG (AP: 3.8, ML: 2.4, DV: 3–3.5) as a recording electrode. Both electrodes were gently lowered and the pathway was stimulated until a standard synaptic signal was obtained. After a 30-min stabilization period, the recording protocol was started. Field potentials were evoked by a 0.2 ms monophasic square current using a stimulator/isolator (A365, WPI, USA); they were then amplified and filtered (between 1 Hz and 3 kHz) using a differential amplifier (DAM 80, WPI, USA) and digitized (10 kHz) and analyzed by a homemade digitizer/software. The amplitude between the first positive upsurge and the most negative point was measured as the PS amplitude. Input/output (I/O) curve of PS amplitude was plotted against different stimulating current magnitudes. A test stimulus with the intensity of 50% of the maximum response obtained from I/O curve was applied every 5 min for 30 min to record baseline synaptic activity. A high-frequency stimulation (HFS), consisting of 10 trains of 20 pulses at 400 Hz with 80% of maximum intensity delivered every 10 s, was used to induce LTP. The criteria for LTP induction were at least 25% increase of the PS amplitude compared to the baseline. After applying HFS, the synaptic responses were recorded every 5 min for 90 min. The paired field potentials were also recorded. They were elicited by paired pulses of stimuli with interpulse intervals (IPIs) ranging from 25 to 370 ms. The paired‐pulse ratio (PPR) was the result of dividing PS amplitude of the second field potential by that of the first one.
Homosynaptic LTD is not readily induced in DG by typical low-frequency stimulation of perforant pathway (Christie and Abraham 1992; Gonzalez et al. 2014). Therefore, we induced heterosynaptic LTD in DG according to the protocol of Christie and Abraham (Christie and Abraham 1992). The heterosynaptic LTD was induced by concurrent stimulation of medial and lateral perforant pathways. Both medial (MPP, AP: − 7.9 mm, L: + 4.2 mm, DV = − 2.5 mm) and lateral (LPP, AP: − 8.0 mm, L: + 5.0 mm, DV: − 2.8 mm) perforant pathways were implanted with Teflon-coated twisted-pair stainless steel wires, as stimulation electrodes. A bipolar twisted-pair stainless steel electrode was situated in the hilar region of DG as the recording electrode. A test stimulus with the intensity of 50% of the maximum response obtained from I/O curve was applied every 2 min for 12 min to record baseline synaptic activity. The MPP was stimulated by eight stimulus trains (a burst of 5 pulses at 100 Hz, repeated at 200 ms intervals) of 2-s duration, with 1-min interval between each. The LPP, which served as the test pathway was stimulated by single pulses of 5 Hz repeated at 200-ms intervals (low-frequency stimulation, LFS), equally spaced between the MPP bursts.
Statistical Analysis
The electrophysiology experiments were repeated three times (n = 7–10), each time by a different expert experimenter who was unaware of the nature of the treatment. The data are expressed as means ± S.E.M. One-way or two-way analysis of variance and post hoc Tukey’s test were used for multiple comparisons. In all cases, a p ≤ 0.05 was considered statistically significant.
Results
RVG Expression in the Hippocampus
The titer of the lentivector was found 2 × 108 T.U./ml. Figure 2a, b show that RVG/eGFP gene was successfully expressed in granule cells 2 weeks after delivery of the lentivector into DG. The nuclear DNA was stained with DAPI (Fig. 2c). Merge picture indicates GFP proteins exclusive localized to the nucleus (Fig. 2c). In the injection side (supra-pyramidal blade of DG), over 90% of the counted DG granule cells expressed bright green fluorescence.
Fig. 2.
DAPI and GFP-fluorescence localization in granule cells of the rat dentate gyrus transfected with plasmids expressing eGFP and RVG proteins. a, b Green cells indicate the successful insertion of the RVG/GFP gene in the granule cells 2 weeks after microinjection of the lentivector into dentate gyrus. c The nuclear DNA was stained with DAPI. Merge picture was used to confirm the co-localization of the nucleus and GFP protein. (Magnification: × 100, scale bar: 50 µm)
RVG Expression in the Hippocampal DG Facilitated Short- and Long-Term Synaptic Plasticity
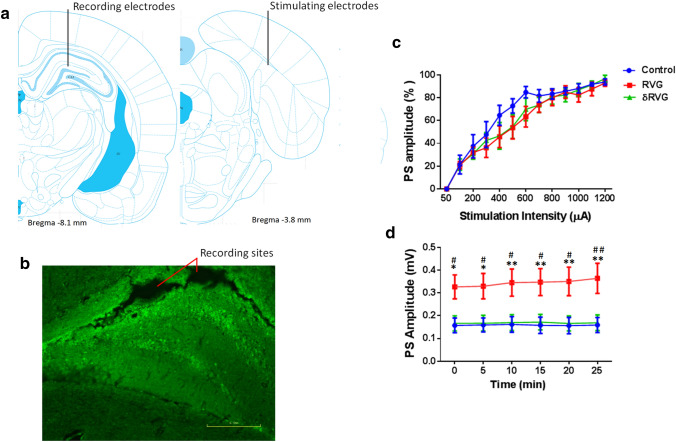
Figure 3a is a schematic representation of dentate gyrus and perforant pathway adapted from atlas of Paxinos and Watson. It shows the position of the stimulating electrode in the perforant pathway and recording electrode in dentate gyrus. Figure 3b displays the position of the bipolar recording electrodes and GFP-expressing cells in dentate gyrus.
Fig. 3.
RVG facilitates basal synaptic transmission in dentate gyrus. a schematic representations of perforant pathway (right) and dentate gyrus (left)
adapted from atlas of Paxinos and Watson showing the position of the stimulating electrodes in the perforant pathway and recording electrodes in dentate gyrus. b A fluorescent image shows location of recording electrodes and transduced cells in the dentate gyrus. c Input and output curves indicate no difference of normalized responses to seven stimulus intensities between groups. d Baseline responses to the 50% maximum of intensity during 30 min are more prominent in rats expressing RVG (n = 10) in dentate gyrus, compared to control (n = 11) and δRVG (n = 10) groups. *p < 0.05 and **p < 0.01 compared to the control group. #p < 0.05 and ##p < 0.01 compared to δRVG group
Synaptic activity was recorded 30 min before applying HFS. Test stimuli were determined from intake output (I/O) curve of PS. PS amplitudes were obtained in DG following stimulation of the perforant pathway with different intensities (Fig. 3c). They were similar for each experimental condition. On the contrary, basal synaptic responses in the DG in response to perforant pathway stimulation with 50% maximum intensity (treatment factor; F (2, 168) = 34.03, p < 0.0001) were greater (> twofold) in RVG (n = 10) group compared to the control (n = 11, p < 0.05) and δRVG (n = 10, p < 0.05) groups. No difference was found between control and δRVG groups (Fig. 3d).
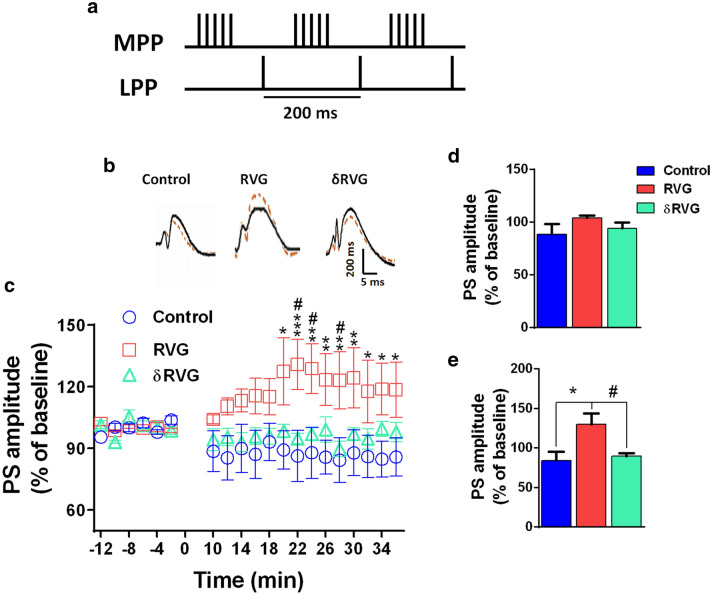
To determine whether the enhanced synaptic transmission observed in RVG group was attributable to presynaptic or postsynaptic mechanisms, we measured paired-pulse facilitation in the DG following double stimulation of perforant pathway with different interpulse intervals. In paired-pulse stimulation, second stimulation of equal intensity normally evokes a greater synaptic response than the first and is used to determine the probability of presynaptic transmitter release. Figure 4a shows representative paired field potential recordings with 75 ms interevent interval. As shown in Fig. 4b and c, at 25 interpulse intervals (IPI), synaptic responses in control group (n = 4) display paired-pulse depression whereas in RVG (n = 8) and δRVG (n = 7) groups, paired-pulse facilitation occurred. Also, synaptic facilitation in other IPIs was more prominent in RVG and δRVG compared to the corresponding synaptic response for control group, indicating that RVG modulates release of excitatory neurotransmitter independent of its PBM site. There was no significant difference between RVG and δRVG in all IPIs.
Fig. 4.
Expression of RVG in the hippocampal dentate gyrus facilitates paired-pulse facilitation. a Representative field potential responses in dentate gyrus evoked by double stimulation of perforant pathway with 70-ms interpulse interval (IPI). b Unlike RVG (n = 8) and δRVG (n = 7), control group (n = 4) showed paired-pulse inhibition in the IPI 25 ms. RVG increases the ratio (the relative PS amplitude of the first and second pulse) in all IPIs but fails to reach significant level. Expression of δRVG (deleted PBM motif) also significantly increases paired-pulse facilitation compared to the control group. In the IPI of 50–120 ms, the PS amplitude of the second response is two times more than that of the first one in the animals expressing δRVG in dentate gyrus. c No difference is observed between IPI of 25 and 70 ms in the control group. However, the paired-pulse ration in RVG and δRVG groups is significantly increased in 70 ms compared to that of 25 ms. *p < 0.05 and **p < 0.01 compared to the control group
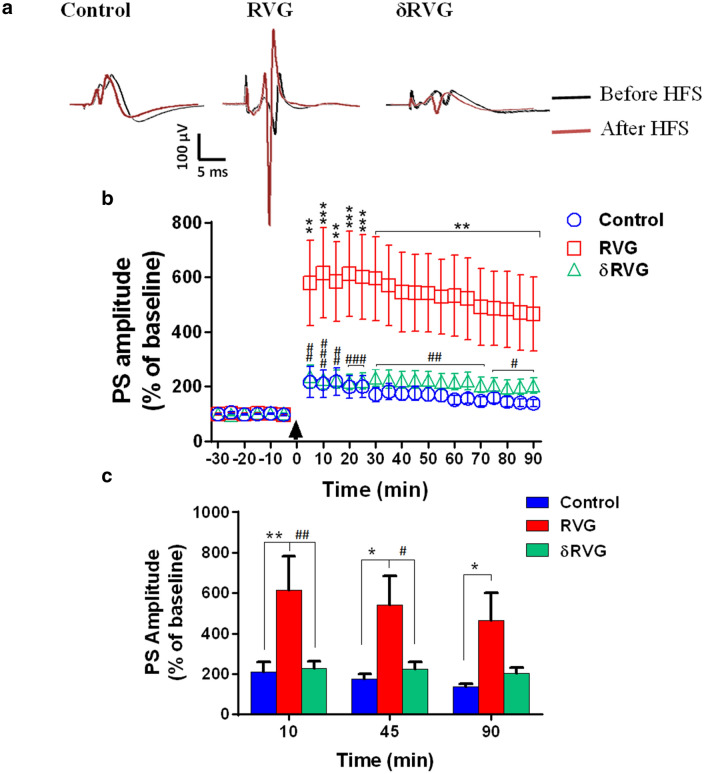
Postsynaptic changes were investigated by extracellular field potential recording. Figure 5a shows representative field potential recordings at baseline and after induction of LTP (post-HFS) at different time points. The time course of PS-LTP induction in the experimental groups is shown in Fig. 5b. Two-way ANOVA revealed a significant effect of treatment [F (1, 270) = 61.59, p < 0.0001, Fig. 5b]. RVG expression (n = 8) in the hippocampal DG augmented PS-LTP amplitude following stimulation of perforant pathway in all time points compared to the control group. However, no difference of PS-LTP amplitude was found between control and δRVG groups indicating that deletion of the PBM motif blocked the facilitating effect of RVG on synaptic plasticity (Fig. 5b). Figure 5c demonstrates the PS amplitudes in time point of 10, 45, and 90 min in different experimental groups demonstrating that RVG expression led to a significant increase of PS amplitude compared to the control and δRVG groups (n = 7 and 10, respectively). Overall, these data indicate that unlike presynaptic function, postsynaptic alteration due to RVG expression in DG is dependent to its PBM sequence.
Fig. 5.
Expression of RVG in the hippocampal dentate gyrus facilitates LTP induction. a Representative field potential responses in dentate gyrus before and after high-frequency stimulation (HFS) of the perforant pathway in all experimental groups. b The time course of PS-LTP induction in the experimental groups. RVG expression facilitates LTP induction in all time points compared to the control group. However, deletion of PBM motif (δRVG group) decreases the synaptic response toward the control level. c PS-LTP amplitude at 10, 45, and 90 min after applying HFS. Expression of RVG significantly increases PS amplitude in all time points compared to the control group. However, the synaptic responses in the δRVG group were similar to the control group. Numbers of subjects in control, RVG, and δRVG groups were 7, 8, and 10, respectively. *p < 0.05, **p < 0.01, and ***p < 0.001 compared to the control group. #p < 0.05, ##p < 0.01, and ###p < 0.001 compared to the RVG group
RVG Blocked Synaptic Long-Term Depression in DG
Figure 6a shows the associative paradigm of long-term depression (LTD) induction in which both MPP (with HFS) and LPP (with LFS) were concurrently stimulated. Two-way ANOVA showed a significant effect of treatment F (2, 352) = 18.19, p < 0.0001, Fig. 5c]. The paradigm induced 15–20% decrease in the PS amplitude in the control group. However, the expression of RVG in DG suppressed LTD induction. No significant difference was found between control (n = 8) and RVG (n = 9) groups in PS amplitude until 20 min time point. Then the difference increased and reached significant levels (p < 0.05). There was no significant difference between control and δRVG groups in PS amplitude indicating that RVG, through its PBM motif, blocks long-term synaptic suppression (Fig. 6c). Figure 6d, e demonstrate the PS amplitudes in time point of 10 and 28 min time points, respectively. No difference was found among groups at the time point of 10 min, but RVG significantly increased PS amplitude (135 ± 14% of baseline) at the 28 min time point. Deletion of PBM site in δRVG group resulted in significant decrease of PS amplitude (88.7 ± 3.7% of baseline) at the time point of 28 min compared to the RVG group
Fig. 6.
RVG expression in the hippocampus blocked long-term synaptic depression. a The associative paradigm for induction of long-term depression (LTD) in which both medial perforant pathway (MPP) and lateral perforant pathway (LPP) were concurrently stimulated by high (bursts of 100 Hz stimuli at 200-ms interburst intervals) and low (single pulses of 5 Hz, equidistantly spaced between the bursts)-frequency stimulation. b Representative field potential responses before and after LTD induction in DG of the experimental groups. c LTD was induced in control group; however, RVG expression suppressed LTD induction in the DG at the all time points (the difference increased and reached to a significant level after 20 min, p < 0.05, compared to the control group). In the δRVG (PBM deletion of RVG) group, LTD was induced in the DG. d, e PS-LTD amplitude at 10 and 28 min after LTD. There was no significant difference among groups at the 10 min time point. However, PS amplitude was significantly increased at the 28 min time point, in the RVG group compared to the control (p < 0.01) and δRVG (p < 0.05) groups. *p < 0.05, **p < 0.01, and ***p < 0.001 compared to the control group. #p < 0.05 compared to the δRVG group
Discussion
The current study provides evidence for interplay between RVG and synaptic function. We found that induction of RVG expression facilitates basic excitatory synaptic transmission and LTP expression in the hippocampal DG through a PBM-dependent manner. Moreover, unlike δRVG, RVG blocked LTD in DG. Furthermore, we found also that RVG strengthens paired-pulse facilitation in DG in all interpulse intervals. These data indicate that RVG may involve both pre- and postsynaptic elements through different mechanisms to alter synaptic function.
RVG which plays a predominant role in the pathogenesis of rabies is a type I glycoprotein which constitutes the trimeric spikes of the virus particle. RVG interacts with cellular receptors, embeds in lipid membrane of the host cell, mediates pH-dependent fusion, and promotes trans-synaptic spread within the CNS (Yousaf et al. 2012). While rabies causes extreme behavioral and neurological disturbances with a 100% fatality rate in humans, relatively mild pathological changes are observed in the infected brain (Fu and Jackson 2005). The underlying mechanism inducing such furious behavioral changes remains poorly understood. Several hypotheses including abnormal synaptic transmission involving serotonin (Bouzamondo et al. 1993), acetylcholine (Dumrongphol et al. 1996), GABA (Ladogana et al. 1994) and dopamine (Fu and Jackson 2005), electrophysiological changes (Gourmelon et al. 1991) and ion channel dysfunction in voltage-gated Na+ and inward rectifier K+ channels (Iwata et al. 1999), or disrupted RNA and protein synthesis (Gosztonyi 1994) have been reported for RABV pathogenesis. Furthermore, myeloid differentiation primary response protein (MyD88)-dependent pathway (Azimzadeh Jamalkandi et al. 2016) and MAST2/phosphatase and tensin homolog (PTEN) signaling pathway (Caillet-Saguy et al. 2015) have been suggested to be involved in the rabies pathogenesis. Also, the virus may induce neuronal dysfunction through acting on fundamental neuronal elements including ion channels to provoke the clinical manifestation rather than neurodegeneration (Lee and Zheng 2010). Although rabies pathogenicity is a multigenic trait, the effect of RABV on the brain function could be attributed to its RVG as a major contributor to the RABV pathogenicity (Pulmanausahakul et al. 2008; Caillet-Saguy et al. 2015; Khan et al. 2019; Lafon 2011). It has been reported that ion channels could be an important target for viruses to alter cellular function (Mankouri et al. 2009). In this line, RABV may also alter neuronal and ultimately cerebral function through action on ion channels. Iwata et al. showed that RABV inhibited voltage-dependent sodium and inward rectifier potassium channels and changed the resting membrane potential resulting in membrane depolarization (Iwata et al. 1999). Street RABV has been shown to induce progressive disappearance of the all stages of sleep waves in the infected mice and increases the duration of waking stages prior to clinical manifestation (Gourmelon et al. 1991) indicating a modulation of neuronal excitability in specific cerebral regions responsible for sleep–waking phases. Furthermore, it has been reported that following infection of rats with RABV, release of neurotransmitters in the hippocampus elevated on the 1st day, reached to a peak on the 3rd day, and then declined on the 5th day (Fu and Jackson 2005). Progressive neurotransmitter release after RABV inoculation could lead to facilitate synaptic transmission and ultimately modulate neuronal excitability which may contribute to clinical symptoms of agitation, hydrophobia, wild aggressiveness, and even seizures in the infected subjects (Hemachudha et al. 1989).
Distribution of the RABV antigen in various cerebral areas is well documented in diverse species, in which hippocampus has been an ideal region for RABV detection (Stein et al. 2010; Song et al. 2013). In the current study, hippocampus was targeted to examine the effect of RVG on synaptic function. Experience-based alteration in synaptic strength underlies memory formation. These changes include persistent long LTP or LTD of synaptic transmission. It has been believed that LTD along with LTP underlies storage of memory (Bear 1996). We found that RVG expression in the hippocampus leads to enhanced synaptic transmission. This synaptic facilitation may be due to increased neurotransmission release from the presynaptic terminals, alteration of the postsynaptic membrane, or both. We found in the present study that RVG could induce both short- and long-term synaptic potentiation. Therefore, it seems that both presynaptic and postsynaptic elements are modulated by RABV. We found that the facilitation on LTP was absent when the C terminal PBM site was missing, indicating that RVG may alter synaptic function in the host cells through its PBM region. We also demonstrate that RVG blocked LTD in DG through its BM site. Both LTP and LTD are the cellular/molecular mechanisms of memory formation. Unlike LTP, LTD is most reliably elicited by elongated low-frequency stimulation, which evokes non-significant postsynaptic depolarization. It is believed that the magnitude of postsynaptic depolarization and resultant increase of intracellular Ca2+ concentration determine whether the synapse undergoes LTP or LTD (Artola and Singer 1993; Cummings et al. 1996). Here, we found that animals expressing RVG in DG express LTP instead of LTD. However, deleting PBM motif restored LTD expression. RVG may alter depolarization state of postsynaptic cells so that they show LTP in response to associative stimulation of LPP and MPP, which normally elicits LTD in DG. In support to this view, it has been reported that depending on the postsynaptic depolarization level in CA1 cells, four trains of a 5 Hz, 2 s stimulus elicit either LTD or LTP (Stanton and Sejnowski 1989). Furthermore, tetanic stimulation has also been reported to elicit NMDA related LTP or LTD in DG depending on the level of postsynaptic depolarization during the tetanus (Xie et al. 1992). Intriguingly, a depolarized resting membrane potential has been reported in neuroblastoma cells infected with rabies virus (Iwata et al. 1999). Yet, further studies using intracellular recording are required to elucidate how RVG changes membrane potential or synaptic inputs (inhibitory and excitatory postsynaptic currents) in DG.
Paired-pulse facilitation (PPF) is a valid procedure for evaluation of short-term plasticity (Castro-Alamancos and Connors 1997; Leung and Fu 1994; Commins and O’Mara 2000; Jackman and Regehr 2017). PPF is one of the well-studied components of short-term plasticity that can easily be assessed in many synaptic model systems by a pair of closely spaced stimuli in what's known as the paired-pulse protocol (Commins and O’Mara 2000). "Facilitation is thought to depend on elevation in presynaptic residual calcium levels that may arise from several mechanisms, including saturation of presynaptic calcium buffers, enhancement of presynaptic calcium currents, and possibly changes in sensitivity of calcium sensor” (Deng and Klyachko 2011), which all result in short-term plasticity. PPF is investigated either by extracellular field recording or by intracellular recording (patch clamping). The field potential records response of a population of neurons, whereas intracellular recording monitors one cell in response to stimulation of a neural pathway. Although data that are obtained by intracellular recording seem more precise, the response may deviate from the physiological state due to cell dialysis by intracellular solution. In the present study, PPFs occur in all interpulse intervals ranging from 25 to 370 ms, in RVG and δRVG groups. Yet, in control group, paired-pulse inhibition (PPI) happened in short (25 ms) and in long interpulse intervals (270 and 370 ms). The facilitation of short-term synaptic transmission (paired-pulse facilitation) in RVG-expressed granular cells of dentate gyrus indicates a decreased probability of neurotransmitter release from presynaptic terminals. This effect of RVG was independent of its PBM site. Fu and Jackson reported that the release of serotonin, norepinephrine, and dopamine was increased in the hippocampus of rat following intranasal inoculation with RABV CVS-24 (Fu and Jackson 2005). On the other hand, it has been shown that the two important synaptic proteins, tripartite motif-containing protein 9 (TRIM9) and α-soluble NSF-attachment protein (α-SNAP), were downregulated in silver-haired bat rabies virus-infected mice whereas infection with attenuated RABV strain (CVS-B2C) did not change the expression level of these two synaptic proteins (Dhingra et al. 2007). In other words, pathogenic RABV strains may blunt synaptic function through downregulation of critical synaptic proteins. However, it should be noted that these proteins were measured in these studies after development of severe paralysis in mice. It may seem that neurotransmitter release is stimulated by the virus in the early stage of the infection but at later stages of infection, neurons are no longer capable of releasing neurotransmitters at the synaptic clefts. Decreased neurotransmitter release in the late stage of disease may be also due to membrane depolarization beyond threshold which results in inactivation of voltage-dependent Na+ and Ca2+ channels and blunt neuronal activation and synaptic transmission. Decreasing neurotransmitter release may also be due to microstructural changes in the infected neurons at the late stage of the disease. In this line, Scott et al. found relatively few changes in the perikarya and neuronal processes in the hippocampi of moribund CVS-infected YFP mice (Scott et al. 2008). Furthermore, disorganized apical dendrites of neurons have been reported in the hippocampus of mice infected with a pathogenic RABV strain, CVS-N2C (Li et al. 2005). It was also shown that RABV did not inhibit gene and protein synthesis in cell culture (Ermine and Flamand 1977; Madore and England 1977) and RABV failed to shut down host cellular DNA, RNA, or protein synthesis in infected brain because of its limited or late onset of cytopathogenic effect (Baer et al. 1990).
RVG may also act through postsynaptic elements to induce synaptic facilitation. In support, we found that RVG, dependent to its PBM site, induced prominent enhanced basal synaptic transmission and LTP, indicating that RVG may alter postsynaptic neuronal mechanism (s) including neuronal excitability rather than presynaptic events. RVG-expressing animals developed LTP instead of LTD in response to LTD-inducing stimulation, which further confirms that RVG may modify postsynaptic membrane potential. In most cases, LTP is dependent on molecular changes including increased AMPA trafficking and NMDA receptors at the postsynaptic membrane. These receptors at the glutamatergic excitatory synapses are accumulated by scaffolding proteins including membrane-associated guanylate kinases (MAGUKs) at the postsynaptic density (PSD). Synaptic incorporation of AMPA receptors containing GluR1 subunit is involved in LTP induction (Hayashi et al. 2000; Shi et al. 2001). Increased GluR1 in the expression site of RVG may secondarily be due to increased neuronal or synaptic activity in response to RVG. In support of this view, recent studies using tracking of individual AMPA receptors showed that the increased synaptic activity could recruit them at synapses (Opazo and Choquet 2011). Interestingly, Khan and his colleagues have recently reported that Neurovita, a C terminal RVG-derived peptide, protects neuronal homeostasis and stimulates neurite outgrowth in several neuron types and the effect was dependent of the PBM site (Khan et al. 2019). In the current study, we found that induction of RVG expression in the dorsal hippocampal DG led to facilitate synaptic activity in a PBM-dependent manner. While no specific electrophysiological study has tested the effect of RABV on synaptic transmission, some evidence indicates that the virus alters neurotransmitter releases in various brain regions, which can be mirrored in multiple clinical manifestations. For instance, hyperexcitability and aggression may be due to increased synaptic activity in the relevant centers including limbic system (amygdala and hypothalamus), or hallucination may be resulted from altered synaptic transmission in the cerebral cortices. Increased activity of areas involved in the autonomic system, such as the hypothalamus, leads to hypersalivation and hyperejaculation in rabid patients (Tian et al. 2019). Furthermore, increased neuronal activity at vital centers in the brainstem, such as the respiratory control center, can functionally alter the pattern of breathing from ramping to the explosive discharge of neurons, hiccups (DelRosso and Hoque 2013), and eventually hypopnea and apnea. Interestingly, the positive role of DG in the control of fear and anxiety has been widely reported by animal and human studies (Cummings et al. 1996; Stanton and Sejnowski 1989; Xie et al. 1992). Our finding on the increased synaptic activity of DG neurons upon infection by RVG might be a physiologic response to counterbalance the fear and anxiety, the typical feature of patients with rabies. Nonetheless, more and specific studies are needed to confirm this suggestion.
Study Limitations
Our study has some limitations. Mechanism (s) by which PBM-deleted RVG modifies presynaptic-dependent short synaptic plasticity without altering postsynaptic-dependent long-term plasticity need to be explored. We found that PPR is increased by PBM-deleted RVG indicating a decrease in neurotransmitter release probability. Studying miniature excitatory postsynaptic currents (mEPSCs) and miniature inhibitory postsynaptic currents (mIPSCs) may clear the action of RVG on the presynaptic sites. In addition, our finding on basal and long-term synaptic changes by RVG but not PBM-deleted RVG indicates possible modification of neuronal depolarization/excitability, which needs to be elucidated by intracellular recording.
Conclusion
To the best of our knowledge, this is the first report indicating that RVG facilitates hippocampal short- and long-term synaptic strength. This finding indicates that RABV may functionally alter synaptic transmission in different cerebral regions through, in part, incorporation of its PBM site. This increased synaptic function might underlie the neurobehavioral disorders seen in rabies disease, or, on the other hand, be a reaction to correct the disturbance of the nervous system induced by the rabies virus. Comprehensive studies are required to elucidate these assumptions and the mechanisms involved in impact of RABV/RVG on synaptic function.
Acknowledgements
This research is supported by Grant No, 868 from Pasteur Institute and is a part of PhD thesis of Soheil Ghassemi.
Author Contributions
HGP and MS involved in study concept and design and study supervision, participated in experimental design and statistical analysis, and obtained funding: SGH, TA, HMD, and SA participated in acquisition of the data. HGP, KA, and MS performed analysis and interpretation of the data. HGP, CP, ML, AG, NN, and MS drafted the manuscript. MS involved in administrative, technical, and material support. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Compliance with Ethical Standards
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical Approval
All animal experiments were carried out in accordance with the Review Board and Ethics Committee of Pasteur Institute (Authorization code 93-0201-785, 22 April 2014) and conform to the European Communities Council Directive 2010-63-EU.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hamid Gholami Pourbadie, Email: h_gholamipour@pasteur.ac.ir.
Mohammad Sayyah, Email: sayyahm2@pasteur.ac.ir.
References
- Artola A, Singer W (1993) Long-term depression of excitatory synaptic transmission and its relationship to long-term potentiation. Trends Neurosci 16(11):480–487 [DOI] [PubMed] [Google Scholar]
- Azimzadeh Jamalkandi S, Mozhgani S-H, Gholami Pourbadie H, Mirzaie M, Noorbakhsh F, Vaziri B, Gholami A, Ansari-Pour N, Jafari M (2016) Systems biomedicine of rabies delineates the affected signaling pathways. Front Microbiol 7:1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer G, Bellini W, Fishbein D (1990) Rhabdoviruses. In: Fields BN, Knipe DM (eds) Virology, 2nd edn. Raven Press, New York, pp 883–930 [Google Scholar]
- Bear MF (1996) A synaptic basis for memory storage in the cerebral cortex. Proc Natl Acad Sci USA 93(24):13453–13459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361(6407):31–39 [DOI] [PubMed] [Google Scholar]
- Bouzamondo E, Ladogana A, Tsiang H (1993) Alteration of potassium-evoked 5-HT release from virus-infected rat cortical synaptosomes. NeuroReport 4(5):555–558 [DOI] [PubMed] [Google Scholar]
- Caillet-Saguy C, Maisonneuve P, Delhommel F, Terrien E, Babault N, Lafon M, Cordier F, Wolff N (2015) Strategies to interfere with PDZ-mediated interactions in neurons: what we can learn from the rabies virus. Prog Biophys Mol Biol 119(1):53–59 [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Connors BW (1997) Distinct forms of short-term plasticity at excitatory synapses of hippocampus and neocortex. Proc Natl Acad Sci USA 94(8):4161–4166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherian S, Singh R, Anjaneya SKP (2015) Rabies glycoprotein: a benefit to the virus, us or both? J Vet Sci 1(1):73–81 [Google Scholar]
- Christie BR, Abraham WC (1992) NMDA-dependent heterosynaptic long-term depression in the dentate gyrus of anaesthetized rats. Synapse 10(1):1–6 [DOI] [PubMed] [Google Scholar]
- Commins S, O’Mara SM (2000) Interactions between paired-pulse facilitation, low-frequency stimulation, and behavioral stress in the pathway from hippocampal area CA1 to the subiculum: dissociation of baseline synaptic transmission from paired-pulse facilitation and depression of the same pathway. Psychobiology 28(1):1–11 [Google Scholar]
- Cummings JA, Mulkey RM, Nicoll RA, Malenka RC (1996) Ca2+ signaling requirements for long-term depression in the hippocampus. Neuron 16(4):825–833 [DOI] [PubMed] [Google Scholar]
- DelRosso L, Hoque R (2013) A case of obstructive sleep apnea, gastroesophageal reflux disease, and chronic hiccups: will CPAP help? J Clin Sleep Med 9(1):92–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng P-Y, Klyachko VA (2011) The diverse functions of short-term plasticity components in synaptic computations. Commun Integr Biol 4(5):543–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra V, Li X, Liu Y, Fu ZF (2007) Proteomic profiling reveals that rabies virus infection results in differential expression of host proteins involved in ion homeostasis and synaptic physiology in the central nervous system. J Neurovirol 13(2):107–117 [DOI] [PubMed] [Google Scholar]
- Dumrongphol H, Srikiatkhachorn A, Hemachudha T, Kotchabhakdi N, Govitrapong P (1996) Alteration of muscarinic acetylcholine receptors in rabies viral-infected dog brains. J Neurol Sci 137(1):1–6 [DOI] [PubMed] [Google Scholar]
- Ermine A, Flamand A (1977) Rna syntheses in BHK21 cells infected by rabies virus. Ann Microbiol 128(4):477–488 [PubMed] [Google Scholar]
- Farzaneh M, Sayyah M, Eshraghi HR, Panahi N, Delavar HM, Pourbadie HG (2018) Transduction efficacy and retrograde movement of a lentiviral vector pseudotyped by modified rabies glycoprotein throughout the trisynaptic circuit of the rat hippocampus. J Gen Med 20(9):e3046 [DOI] [PubMed] [Google Scholar]
- Fourcaudot E, Gambino F, Casassus G, Poulain B, Humeau Y, Lüthi A (2009) L-type voltage-dependent Ca 2+ channels mediate expression of presynaptic LTP in amygdala. Nat Neurosci 12(9):1093–1095 [DOI] [PubMed] [Google Scholar]
- Fu ZF, Jackson AC (2005) Neuronal dysfunction and death in rabies virus infection. J Neurovirol 11(1):101–106 [DOI] [PubMed] [Google Scholar]
- Gonzalez J, Morales IS, Villarreal DM, Derrick BE (2014) Low-frequency stimulation induces long-term depression and slow onset long-term potentiation at perforant path-dentate gyrus synapses in vivo. J Neurophysiol 111(6):1259–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosztonyi G (1994) Reproduction of lyssaviruses: ultrastructural composition of lyssavirus and functional aspects of pathogenesis. Curr Top Microbiol Immunol 187:43–68 [DOI] [PubMed] [Google Scholar]
- Gourmelon P, Briet D, Clarençon D, Court L, Tsiang H (1991) Sleep alterations in experimental street rabies virus infection occur in the absence of major EEG abnormalities. Brain Res 554(1–2):159–165 [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Shi S-H, Esteban JA, Piccini A, Poncer J-C, Malinow R (2000) Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science 287(5461):2262–2267 [DOI] [PubMed] [Google Scholar]
- Hemachudha T, Tirawatnpong S, Phanthumchinda K (1989) Seizures as the initial manifestation of paralytic rabies. J Neurol Neurosurg Psychiatry 52(6):808–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata M, Komori S, Unno T, Minamoto N, Ohashi H (1999) Modification of membrane currents in mouse neuroblastoma cells following infection with rabies virus. Br J Pharmacol 126(8):1691–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata M, Unno T, Minamoto N, Ohashi H, Komori S (2000) Rabies virus infection prevents the modulation by α2-adrenoceptors, but not muscarinic receptors, of Ca2+ channels in NG108-15 cells. Eur J Pharmacol 404(1–2):79–88 [DOI] [PubMed] [Google Scholar]
- Jackman SL, Regehr WG (2017) The mechanisms and functions of synaptic facilitation. Neuron 94(3):447–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AC (2010) Why does the prognosis remain so poor in human rabies? Expert Rev Anti Infect Ther 8(6):623–625 [DOI] [PubMed] [Google Scholar]
- Khan Z, Terrien E, Delhommel F, Lefebvre-Omar C, Bohl D, Vitry S, Bernard C, Ramirez J, Chaffotte A, Ricquier K (2019) Structure-based optimization of a PDZ binding motif within a viral peptide stimulates neurite outgrowth. J Biol Chem 294(37):13755–13768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladogana A, Bouzamondo E, Pocchiari M, Tsiang H (1994) Modification of tritiated γ-amino-n-butyric acid transport in rabies virus-infected primary cortical cultures. J Gen Virol 75(Pt 3):623–627 [DOI] [PubMed] [Google Scholar]
- Lafon M (2011) Evasive strategies in rabies virus infection. Adv Virus Res 79:33–53 [DOI] [PubMed] [Google Scholar]
- Lee H-J, Zheng JJ (2010) PDZ domains and their binding partners: structure, specificity, and modification. Cell Commun Signal 8(1):8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung LS, Fu X-W (1994) Factors affecting paired-pulse facilitation in hippocampal CA1 neurons in vitro. Brain Res 650(1):75–84 [DOI] [PubMed] [Google Scholar]
- Li X-Q, Sarmento L, Fu ZF (2005) Degeneration of neuronal processes after infection with pathogenic, but not attenuated, rabies viruses. J Virol 79(15):10063–10068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore HP, England JM (1977) Rabies virus protein synthesis in infected BHK-21 cells. J Virol 22(1):102–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankouri J, Dallas ML, Hughes ME, Griffin SD, Macdonald A, Peers C, Harris M (2009) Suppression of a pro-apoptotic K+ channel as a mechanism for hepatitis C virus persistence. Proc Natl Acad Sci USA 106(37):15903–15908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opazo P, Choquet D (2011) A three-step model for the synaptic recruitment of AMPA receptors. Mol Cell Neurosci 46(1):1–8 [DOI] [PubMed] [Google Scholar]
- Prehaud C, Lay S, Dietzschold B, Lafon M (2003) Glycoprotein of nonpathogenic rabies viruses is a key determinant of human cell apoptosis. J Virol 77(19):10537–10547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulmanausahakul R, Li J, Schnell MJ, Dietzschold B (2008) The glycoprotein and the matrix protein of rabies virus affect pathogenicity by regulating viral replication and facilitating cell-to-cell spread. J Virol 82(5):2330–2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott CA, Rossiter JP, Andrew RD, Jackson AC (2008) Structural abnormalities in neurons are sufficient to explain the clinical disease and fatal outcome of experimental rabies in yellow fluorescent protein-expressing transgenic mice. J Virol 82(1):513–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S-H, Hayashi Y, Esteban JA, Malinow R (2001) Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell 105(3):331–343 [DOI] [PubMed] [Google Scholar]
- Song Y, Hou J, Qiao B, Li Y, Xu Y, Duan M, Guan Z, Zhang M, Sun L (2013) Street rabies virus causes dendritic injury and F-actin depolymerization in the hippocampus. J Gen Virol 94(2):276–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton PK, Sejnowski TJ (1989) Associative long-term depression in the hippocampus induced by hebbian covariance. Nature 339(6221):215–218 [DOI] [PubMed] [Google Scholar]
- Stein L, Rech R, Harrison L, Brown C (2010) Immunohistochemical study of rabies virus within the central nervous system of domestic and wildlife species. Vet Pathol 47(4):630–633 [DOI] [PubMed] [Google Scholar]
- Tian Z, Chen Y, Yan W (2019) Clinical features of rabies patients with abnormal sexual behaviors as the presenting manifestations: a case report and literature review. BMC Infect Dis 19(1):679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordo N, Kouknetzoff A (1993) The rabies virus genome: an overview. Onderstepoort J Vet Res 60(4):263–269 [PubMed] [Google Scholar]
- Tsiang H (1993) Pathophysiology of rabies virus infection of the nervous system. Adv Virus Res 42:375–412 [DOI] [PubMed] [Google Scholar]
- Xie X, Berger TW, Barrionuevo G (1992) Isolated NMDA receptor-mediated synaptic responses express both LTP and LTD. J Neurophysiol 67(4):1009–1013 [DOI] [PubMed] [Google Scholar]
- Yousaf MZ, Qasim M, Zia S, Ashfaq UA, Khan S (2012) Rabies molecular virology, diagnosis, prevention and treatment. Virol J 9(1):1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]