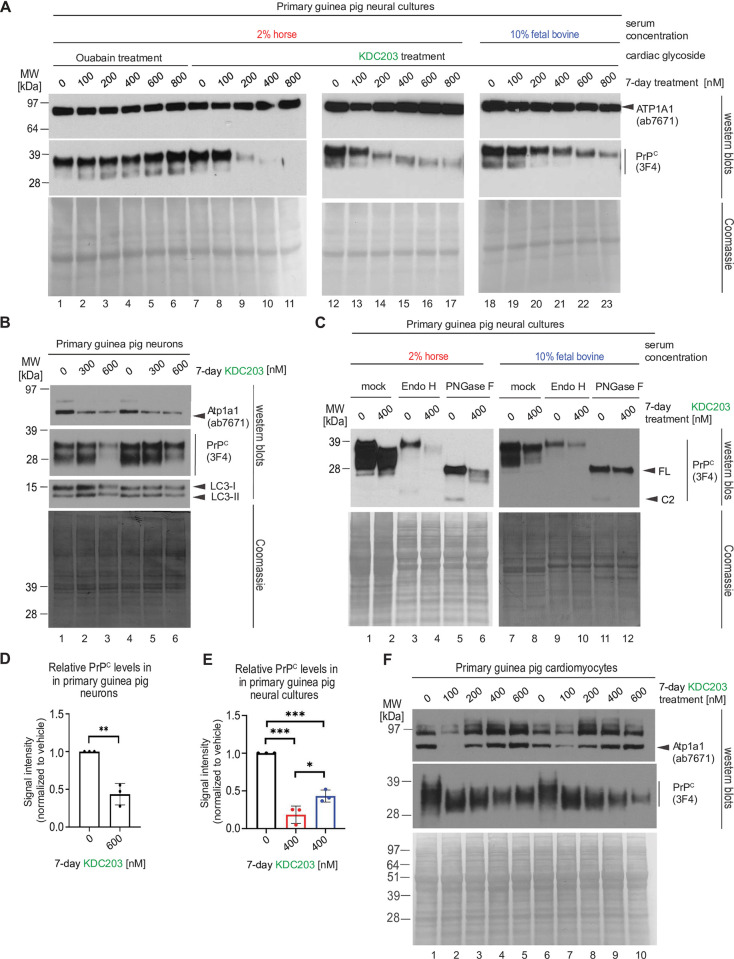
Fig 3. KDC203 lowers PrPC levels in primary cultures of guinea pig neurons, astrocytes, or cardiomyocytes.
(A) KDC203, but not Ouabain, lowers steady-state PrPC levels in primary guinea pig neural cultures, with the PrPC lowering potency enhanced in media containing 2% horse serum than 10% fetal bovine serum. (B) Primary neurons respond to KDC203 with the lowering of steady-state Atp1a1 and PrPC protein levels. LC3-directed western blotting does not reveal the molecular autophagy signature even when cells were exposed to 600 nM KCD203 levels. (C) 7-day KDC203 treatment of primary guinea pig astrocytes led to a reduction in steady-state PrPC levels and a downward shift in its apparent molecular weight that was more pronounced in 2% horse serum than 10% fetal bovine serum. The subsequent digestion with endoglycosidase H (Endo H) or PNGase F failed to reveal a shift in the apparent MW of PrPC in response to Endo H yet documented a profound shift upon PNGase F digestion, consistent with KDC203 at 400 nM having had no impact on the ability of PrPC to reach the cell surface. Note that this analysis did not consider relative signal intensities prior to and after digestion (likely caused by a reduced propensity of PrPC to transfer to the western blot transfer upon removal of its N-glycans). (D) Densitometric western blot quantitation of steady-state PrPC levels in primary guinea pig neurons following 7-day KDC203 treatment, including the results shown in Panel B. (E) Densitometric western blot quantitation of steady-state PrPC levels in primary guinea pig neural cell cultures that were for 7 days exposed to vehicle or 400 nM KDC203 in the presence of 2% horse serum (red bar) or 10% fetal bovine serum (blue bar), including the results shown in Panels A and C. (F) 7-day KDC203 treatment of primary guinea pig cardiomyocytes led to a bimodal shift in the steady-state levels of Atp1a1 that was most pronounced at 100 nM treatment concentrations. In contrast, the signal intensities of PrPC western blot bands declined in a KDC203 concentration-dependent manner that was accompanied by an increase in their mobility in samples derived from KDC203-treated cardiomyocytes. Two biological replicates were included for each KDC203 concentration to document the reproducibility of these complex changes to PrPC and Atp1a1 signals in this cell model.