Fig. 1.
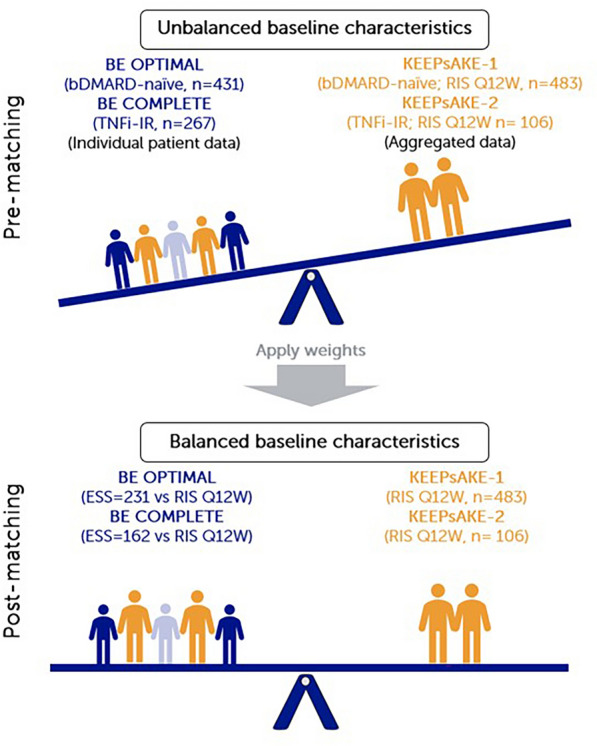
Summary of MAIC matching. MAICs use IPD from trials of one treatment to match baseline aggregate statistics reported from trials of another treatment. Using propensity score weighting techniques to balance trial population characteristics, indirect comparisons can be made. Trial populations adjusted for age, sex, disease duration, MTX use, HAQ-DI, BSA ≥ 3%, SJC and TJC. bDMARD biologic disease-modifying anti-rheumatic drug, BSA body surface area, ESS effective sample size, HAQ-DI Health Assessment Questionnaire–Disability Index, IPD individual patient data, MAIC matching-adjusted indirect comparison, MTX methotrexate, Q12W every 12 weeks, RIS risankizumab, SJC swollen joint count, TJC tender joint count, TNFi-IR tumor necrosis factor inhibitor-inadequate response or intolerant
