Fig. 2.
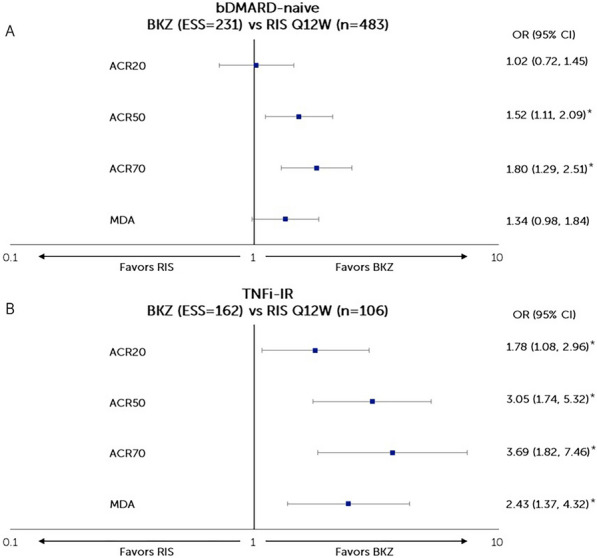
Matching-adjusted odds ratio comparison of bimekizumab vs risankizumab at Week 52 (NRI). A BKZ 160 mg Q4W vs RIS 150 mg Q12W in patients with PsA who were bDMARD naïve, B BKZ 160 mg Q4W vs RIS 150 mg Q12W in patients with PsA who were TNFi-IR. *Statistical significance. Figure shows a logarithmic scale. ACR American College of Rheumatology, ACR20/50/70 at least a 20/50/70% improvement according to the ACR response criteria, bDMARD biologic disease-modifying anti-rheumatic drugs, BKZ bimekizumab, CI confidence interval, ESS effective sample size, MDA minimal disease activity, NRI non-responder imputation, OR odds ratio, PsA psoriatic arthritis, Q4W every 4 weeks, Q12W every 12 weeks, RIS risankizumab, TNFi-IR tumor necrosis factor inhibitor-inadequate response or intolerant
