Figure 3.
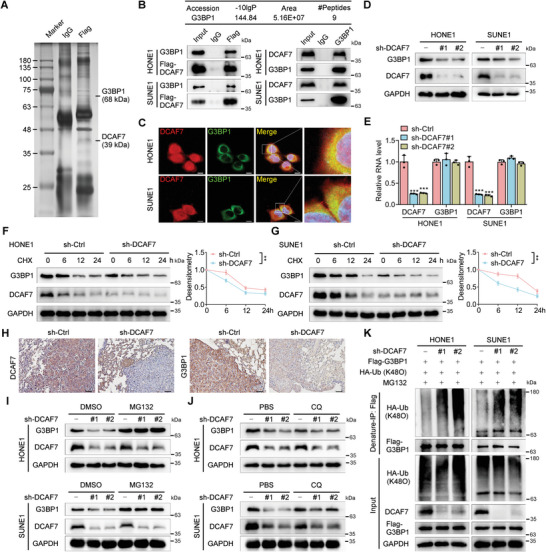
Knockdown of DCAF7 facilitates the degradation of G3BP1 by increasing its K48‐linked polyubiquitination. A) Flag‐DCAF7‐ or vector‐transfected SUNE1 cells were subjected to immunoprecipitation with an anti‐Flag antibody, followed by SDS‒PAGE and silver staining of proteins. The proteins in the bands were analyzed by MS. B) The mass spectrometry results identifying G3BP1 as a potential binding partner of DCAF7 (top). Immunoprecipitation (IP) with an anti‐Flag or anti‐G3BP1 antibody and immunoblot analysis (IB) of G3BP1, Flag or DCAF7 expression in HONE1 and SUNE1 cells transfected with or without Flag‐DCAF7 (bottom). C) Confocal microscopy images showing the colocalization of DCAF7 and G3BP1 in HONE1 and SUNE1 cells. Scale bars: 10, 2 µm (magnified graphs). D,E) Western blotting and RT‒qPCR were used to measure the protein and mRNA levels of G3BP1 in HONE1 and SUNE1 cells following DCAF7 knockdown. Mean (n = 3) ± s.d. One‐way ANOVA, *** p < 0.001. F,G) IB of G3BP1, DCAF7 and GAPDH (left) in HONE1 and SUNE1 cells transduced with sh‐DCAF7 or sh‐control following CHX treatment for the indicated times. Plots showing the normalized G3BP1 levels are also presented (right). Mean (n = 3) ± s.d. Two‐way ANOVA, ** p < 0.01. H) IHC staining for DCAF7 and G3BP1 in the lung metastatic nodules of the mouse model. Scale bar: 50 µm. I,J) IB of G3BP1, DCAF7 and GAPDH in HONE1 and SUNE1 cells transduced with sh‐control or sh‐DCAF7 following treatment with MG132 (10 µm) or CQ (50 µm). K) Denaturing IP (with an anti‐Flag antibody) and IB of HA, Flag, DCAF7 and GAPDH in HONE1 and SUNE1 cells transfected with the indicated plasmids following MG132 treatment (10 µm, 6 h). The unprocessed images of the blots are shown in Figure S10 (Supporting Information).
