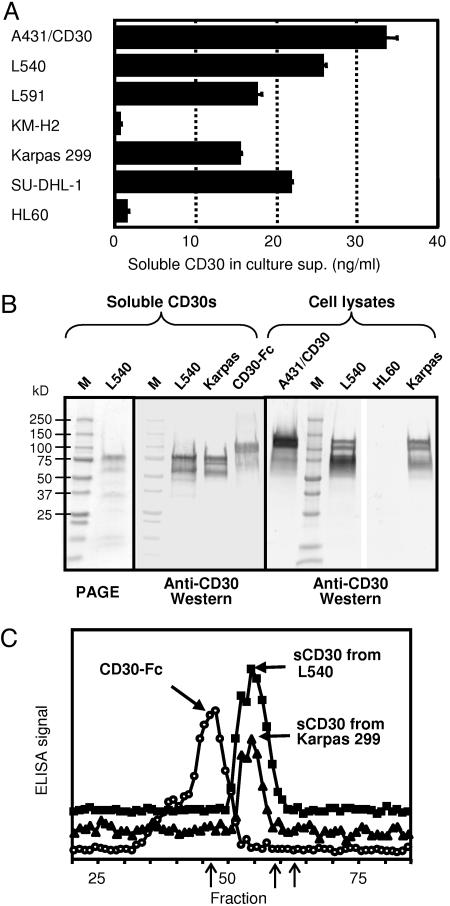
Fig. 1.
Characterization of soluble CD30s produced by cell lines. (A) Soluble CD30 levels measured by a sandwich ELISA in the culture supernatants of various cell lines. Cells were inoculated at 4 × 105 per ml and cultured for 36 h. Standard deviations of triplicate measurement are shown as bars. (B) SDS/PAGE and Western blot analysis of the soluble CD30 and cell lysates. Two micrograms of affinity-purified soluble CD30 protein were separated in 4–20% gradient SDS/PAGE gel under reducing conditions (Left). Twenty nanograms of purified proteins or 40 μg of total cell lysates were used for the Western blotting (Center and Right). (C) Size-exclusion chromatography analysis of soluble CD30s and CD30-Fc. One hundred nanograms of each protein in 0.5% BSA was separated on a TSK SW4000 column, and 0.2-ml fractions were measured by an anti-CD30 sandwich ELISA. From the standard proteins (three arrows in the fraction line: Fr.46.5 = 670,000, Fr.9 = 158,000, and Fr.63 = 44,000), the molecular masses of the major peaks of CD30-Fc and sCD30s were estimated as 645 and 317 kDa, respectively.