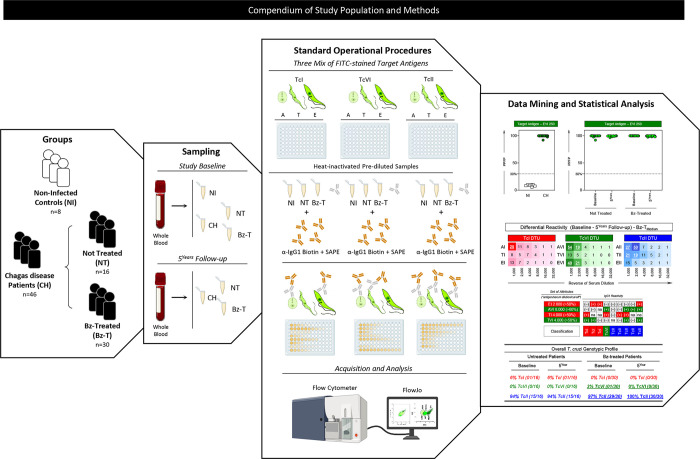
Fig 1. Compendium of study population and methods.
An overview of study groups, sampling, standard procedures, data mining and statistical analysis summarizes the compendium of study population and methods. The study groups comprised a non-probabilistic convenience sampling from archival biorepository, including 54 adult subjects, both sexes, referred as: chronic Chagas disease patients (CH, n = 46) and non-infected healthy subjects (NI, n = 8). CH were further classified into two subgroups, named: Not Treated (NT, n = 16) and Benznidazole Treated (Bz-T, n = 30). Serum specimens from CH were collected at two time points: at Study Baseline and at 5Years Follow-up. Chagas-Flow ATE IgG1 standard procedure was carried out as previously reported by Alessio et al. (2020) [24]. FITC-labeled parasites (ATE target antigen mix) were incubated with heat-inactivated pre-diluted samples followed by addition of second step reagents (biotin-conjugated anti-human IgG1 antibody plus streptavidin phycoerytrin–SAPE). TcI, TcVI or TcII Chagas-Flow ATE IgG1 were performed in simultaneous assays. Parasite suspensions were acquired in a FACSCalibur flow cytometer (BD Bioscience, San Diego, CA, USA). Distinct approaches were used for data mining and statistical analysis, including: IgG1 reactivity, expressed as percentage of positive fluorescent parasites (PPFP) to specific target antigens; differential median reactivity (Study Baseline– 5Years Follow-up) of CH subgroups (NT and Bz-T) and changes in genotype-specific serological profiles upon Bz-treatment were assessed using specific sets of TcI, TcVI and TcII target antigens reported in reactivity boards.