Abstract
BACKGROUND:
Chronic inflammation initiated by inflammatory monocytes underlies the pathogenesis of atherosclerosis. However, approaches that can effectively resolve chronic low-grade inflammation targeting monocytes are not readily available. The small chemical compound 4-phenylbutyric acid (4-PBA) exhibits broad anti-inflammatory effects in reducing atherosclerosis. Selective delivery of 4-PBA reprogrammed monocytes may hold novel potential in providing targeted and precision therapeutics for the treatment of atherosclerosis.
METHODS:
Systems analyses integrating single-cell RNA sequencing and complementary immunologic approaches characterized key resolving characteristics as well as defining markers of reprogrammed monocytes trained by 4-PBA. Molecular mechanisms responsible for monocyte reprogramming were assessed by integrated biochemical and genetic approaches. The intercellular propagation of homeostasis resolution was evaluated by coculture assays with donor monocytes trained by 4-PBA and recipient naive monocytes. The in vivo effects of monocyte resolution and atherosclerosis prevention by 4-PBA were assessed with the high-fat diet-fed ApoE−/− mouse model with IP 4-PBA administration. Furthermore, the selective efficacy of 4-PBA-trained monocytes was examined by IV transfusion of ex vivo trained monocytes by 4-PBA into recipient high-fat diet-fed ApoE−/− mice.
RESULTS:
In this study, we found that monocytes can be potently reprogrammed by 4-PBA into an immune-resolving state characterized by reduced adhesion and enhanced expression of anti-inflammatory mediator CD24. Mechanistically, 4-PBA reduced the expression of ICAM-1 (intercellular adhesion molecule 1) via reducing peroxisome stress and attenuating SYK (spleen tyrosine kinase)-mTOR (mammalian target of rapamycin) signaling. Concurrently, 4-PBA enhanced the expression of resolving mediator CD24 through promoting PPARγ (peroxisome proliferator-activated receptor γ) neddylation mediated by TOLLIP (toll-interacting protein). 4-PBA-trained monocytes can effectively propagate anti-inflammation activity to neighboring monocytes through CD24. Our data further demonstrated that 4-PBA-trained monocytes effectively reduce atherosclerosis pathogenesis when administered in vivo.
CONCLUSIONS:
Our study describes a robust and effective approach to generate resolving monocytes, characterizes novel mechanisms for targeted monocyte reprogramming, and offers a precision therapeutics for atherosclerosis based on delivering reprogrammed resolving monocytes.
Keywords: atherosclerosis; immunity, innate; inflammation; monocytes; therapeutics
Novelty and Significance.
What Is Known?
Inflammatory monocytes trained by metabolic danger signals such as oxLDL (oxidized low-density lipoprotein) exhibit elevated recruitment and adhesion to vasculature, which can exacerbate atherosclerosis pathogenesis.
4-phenylbutyric acid (4-PBA) training can generate monocytes with general anti-inflammatory features.
Systemic administration of 4-PBA, a derivative compound of butyric acid, can reduce systemic inflammation and alleviate atherosclerosis pathogenesis.
What New Information Does This Article Contribute?
TRAM (Trif-related adapter molecule) serves as a generic stress sensor for oxLDL in initiating sustained low-grade inflammatory monocyte memory represented by elevated levels of ICAM-1 (intercellular adhesion molecule 1) and chemokine ligand 5 (CCL5), which can be effectively attenuated by the treatment with 4-PBA.
Anti-inflammatory resolving monocytes trained by 4-PBA are less adhesive, with a salient signature of elevated CD24 expression, and can propagate its anti-inflammatory features to neighboring immune cells. 4-PBA reprograms anti-inflammatory resolving monocytes through Tollip-mediated PPARγ (peroxisome proliferator-activated receptor γ) activation.
When transfused in vivo, 4-PBA–trained monocytes can alleviate inflammation and propagate immune homeostasis within vasculature tissue niche, and reduce atherosclerosis pathogenesis.
Monocytes have divergent roles in either promoting or reducing the pathogenesis of atherosclerosis, likely dependent upon their polarization states. Exact mechanisms of monocyte polarization triggered by oxLDL, as well as effective intervention of monocyte inflammatory polarization are not well characterized. This study demonstrates that 4-PBA can effectively train resolving monocytes through Tollip-mediated PPARγ activation and CD24 expression. CD24 on resolving monocytes are responsible for propagating anti-inflammatory homeostasis to neighboring immune cells within the vasculature tissues, and reducing atherosclerosis. These findings suggest that monocytes trained by 4-PBA may provide a novel cell-based therapeutic approach for atherosclerosis.
Meet the First Author, see p 804
Atherosclerosis is a complex chronic inflammatory disease culminating in the buildup of lipid-laden plaques within arterial vessels. Existing translational efforts are largely focused on developing chemical- or biologic-based therapies that target metabolic and inflammatory processes.1–3 However, these approaches suffer from inherent drawbacks such as limited delivery precision into inflamed tissues and unintended side effects. As an alternative approach, mobilizing immune cells naturally equipped with effective physiological tropism into inflamed tissues may hold powerful therapeutic potentials in circumventing the drawbacks associated with molecule-based therapies.
To fully harness immune cell-based therapies, fundamental studies navigating the complex dynamics of atherosclerosis-associated immune cells are urgently needed. Emerging studies have identified monocytes as one of the most relevant immune cells involved in both the progression and resolution of atherosclerosis.4–7 The initial polarization of inflammatory monocyte subsets (eg, Ly6Chi murine monocytes or CD14+; CD16+ intermediate human monocytes) may serve as a key trigger for tissue infiltration, adhesion, and subsequent foamy macrophage formation.8,9 Strategies that can prevent the initial expansion of inflammatory monocytes would assist in the attenuation of atherosclerosis pathogenesis. Intriguingly, in vivo studies have also revealed that certain subsets of Ly6Chi monocytes with limited proliferation potential can serve as precursors for anti-inflammatory resolving monocytes beneficial for atherosclerosis regression.10–13 Our recent in vitro studies with single-cell RNA sequencing pseudotime analyses further validated these in vivo observations by revealing the presence of distinct subsets of proliferative monocytes adopting either inflammatory or resolving properties.14,15 Following dedifferentiation into a proliferative Ly6Chi high state,6,14,15 monocytes bifurcate into either an inflamed state with the sustained stimulation of a low-grade inflammatory signal6 or remain in an anti-inflammatory proliferative state.14,15 A clear characterization and derivation of resolving monocytes may facilitate future therapeutic development of monocyte-based precision therapies against atherosclerosis.
We recently reported that monocytes trained with the small chemical compound 4-phenylbutyric acid (4-PBA) are arrested at the proliferative resolving state.14,15 4-PBA is a potent peroxisome activator and can induce the expression of a PPAR-mediated peroxisome synthesis program as well as other anti-inflammatory mediators.16–18 Independent studies reported that the administration of 4-PBA into whole animals can reduce the progression of experimental atherosclerosis.19–21 To avoid the potential toxic effects of the systemic 4-PBA administration, we tested the approach of employing 4-PBA-trained monocytes in delivering targeted therapeutics against atherosclerosis. Through integrated in vitro and in vivo studies, we defined key resolving characteristics of 4-PBA programmed monocytes, their underlying molecular mechanisms, and their therapeutic potential in attenuating atherosclerosis.
METHODS
Data Availability
All supporting data of this study are available from the corresponding author upon reasonable request. RNA-seq data have been uploaded to the NCBI Gene Expression Omnibus and are accessible under accession number GSE160450. Methylation profiling data have been uploaded to the NCBI Gene Expression Omnibus and are accessible under accession number GSE243358. For detailed experimental methods, materials, and statistical analysis, please see the Supplemental Material and the Major Resource Table.
RESULTS
Monocytes Trained With 4-PBA Exhibit Potent Anti-Inflammatory Resolving Characteristics
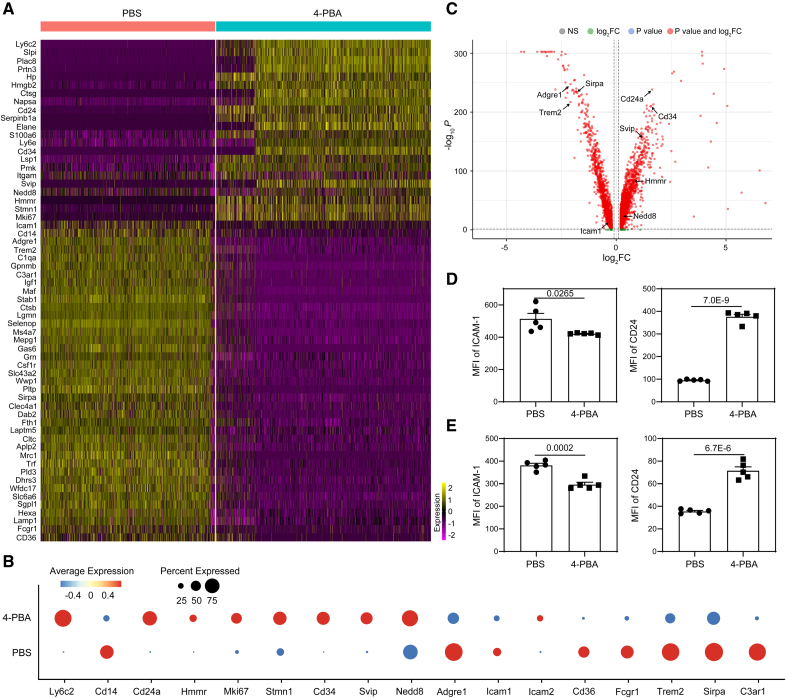
Administration of 4-PBA was independently shown to be beneficial in reducing atherosclerosis,19–21 indicating that 4-PBA may serve as a promising atheroprotective agent. Our in vitro studies reveal that monocytes treated with 4-PBA are arrested in an anti-inflammatory state, suggesting monocytes may be responsible for atheroprotective benefits of 4-PBA in animal models. To better define and harness the therapeutic potential of resolving monocytes programed by 4-PBA, we re-analyzed the gene expression profile of monocytes treated with 4-PBA and monitored for key signatures indicative of monocyte differentiation and activation. As shown in Figure 1, monocyte subsets trained by 4-PBA express monocytic markers such as Cd34 and have reduced expression of mature macrophage markers such as Adgre1 (F4/80), consistent with morphological observation (Figure S1A) demonstrating the monocytic nature of 4-PBA–trained monocytes as compared with control monocyte cultures. Our scRNAseq data also suggest that 4-PBA–trained monocyte subsets express higher levels of genes representative of elevated proliferative potentials such as Hmmr, Mki67, and Stmn1 (Figure 1A through 1C). In contrast to traditional culture media supplemented with L929 supernatant which includes additional growth and differentiation factors in addition to M-CSF (macrophage colony-stimulating factor), low-dose pure M-CSF selectively maintains the survival of monocyte-like cells without inducing cellular activation and differentiation.6,22 We have systematically characterized the 5-day culture model of murine bone marrow monocytes supplemented by low-dose M-CSF, achieving robust maintenance of >99% monocytes by the end of the 5-day culture period.6 To further validate the culture system, we did comparative flow analyses of culture monocytes with fully differentiated macrophages as well as dendritic cells (DCs). Both PBS- and 4-PBA-trained cells cultured for 5 days supplemented with M-CSF exhibited high levels of the myeloid marker CD11b but were completely negative for the progenitor marker c-kit, indicating an absence of hematopoietic progenitor cell contamination within our culture system (Figure S2A). As compared with isolated peritoneal resident macrophages, both PBS- and 4-PBA-trained cells expressed high levels of monocyte marker CD93 and low levels of macrophage marker CD105,23 further confirming that our cultured cells were monocyte-like cells rather than fully differentiated macrophages (Figure S2A). Furthermore, compared with bone marrow–derived DCs, PBS- or 4-PBA–trained monocytes were negative for the DC marker CD11c and expressed low levels of MHCII, indicating that DCs were not generated in the culture system (Figure S2B).
Figure 1.
Treatment with 4-phenylbutyric acid (4-PBA) induces anti-inflammatory resolving characteristics of monocytes. A through D, Bone marrow–derived monocytes (BMMs) from wild-type (WT) C57 BL/6 mice were cultured in vitro with macrophage colony-stimulating factor (M-CSF; 10 ng/mL) in the presence 4-PBA (1 mmol/L) or PBS for 5 days. Single-cell RNA sequencing (scRNAseq) was performed, and data sets were processed to compare naive murine monocytes with monocytes trained by 4-PBA. A, Heatmaps demonstrating representative genes differentially expressed in different clusters of monocytes challenged with 4-PBA. B, Dot plot comparison of representative genes differentially expressed between PBS- vs 4-PBA–trained monocytes. C, Volcano plot showing differentially expressed genes between PBS- vs 4-PBA–trained monocytes. D, Surface expression of ICAM-1 (intercellular adhesion molecule 1) and CD24 on CD11b+ monocytes cultured was analyzed by flow cytometry. E, Peripheral blood mononuclear cells (PBMCs) isolated from healthy human individuals were cultured in vitro with M-CSF (100 ng/mL) in the presence 4-PBA (1 mmol/L) or PBS for 2 days. Surface expression of ICAM-1 and CD24 on total monocytes (including CD14hi, CD16hi, and intermediate monocytes) was analyzed by flow cytometry. Data in D and E (n=5 for each group, biological replicates) were analyzed with Student t test. Error bars represent means±SEM.
We next examined pro- and anti-inflammatory gene signatures of trained monocytes by 4-PBA and observed lower levels of adhesion molecules such as ICAM-1 (intercellular adhesion molecule 1) and drastically elevated anti-inflammatory mediators such as CD24. ICAM-1 plays a pivotal role as a proinflammatory biomarker that facilitates monocyte adhesion to aortic endothelial cells and contributes to the development of atherosclerotic plaques.24,25 Functionally, we validated that 4-PBA-trained monocytes exhibit significantly reduced adhesion capacity and elevated migratory potential (Figure S1B and S1C; Videos S1 and S2). We further evaluated the foam cell formation potential in monocytes trained by 4-PBA. PBS- and 4-PBA-trained monocytes were incubated with fluorescently conjugated oxLDL (oxidized low-density lipoprotein), and the accumulation of intracellular oxLDL was visualized by fluorescence microscopy and quantified by flow cytometry. We observed that 4-PBA treatment significantly reduced the intracellular oxLDL accumulation, suggesting that the form cell formation was remarkably suppressed in 4-PBA-trained monocytes (Figure S3).
The expression of CD24 is found on the surface of various hematopoietic cell populations and thus CD24 expression is higher in progenitor cells and less-differentiated cells as compared with terminally differentiated cells.26 CD24 has also been well documented to interact with Siglec-10 to suppress inflammatory responses of innate immune cells to infection, sepsis, and chronic inflammatory diseases.27 Based on the clues learned from the scRNAseq analyses, we further independently validated the expression of ICAM-1 and CD24 in 4-PBA-treated cells at the protein levels, with our previously established murine monocyte culture system.16,28 In line with our single-cell RNA sequencing results, flow cytometry demonstrated that treatment with 4-PBA for 5 days significantly reduced ICAM-1 expression on murine bone marrow–derived monocytes (BMMs) compared with PBS control cells (Figure 1D). In terms of CD24, monocytes trained by 4-PBA were 100% homogenously CD24 ++ and clearly separated from the populations of control monocytes (Figure S2C).
To validate the translational relevance of these findings, we tested whether 4-PBA can elicit similar proresolving characteristics in human primary monocytes. We cultured human peripheral blood mononuclear cells with either PBS or 4-PBA and observed a significant reduction of ICAM-1 and elevation of CD24 in 4-PBA-treated cells (Figure 1E). Our data reveal that 4-PBA programmed monocytes adopt key resolving signatures of monocytes with uniformly higher levels of CD24 expression.
4-PBA Injection Reprograms Monocytes In Vivo and Reduces Atherosclerosis
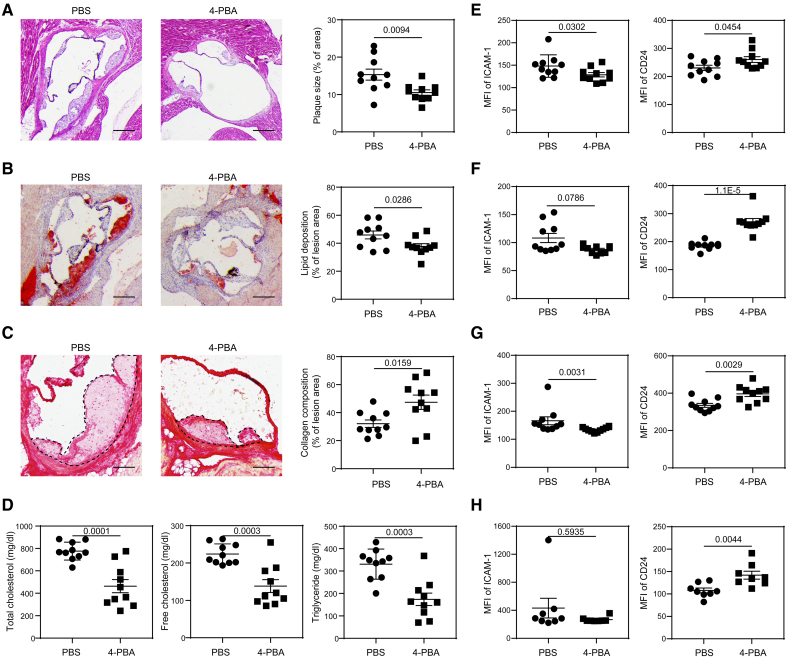
We next tested whether these key signatures of resolving monocytes can be recapitulated in mice treated with 4-PBA in vivo. We initially confirmed that administration of 4-PBA may alleviate atherosclerosis progression in our experimental mouse model. Whereas previous studies administrated 4-PBA through drinking water,19,20 we elected to treat high-fat diet (HFD)–fed ApoE−/− mice with 4-PBA via intraperitoneal injection (100 mg/kg body weight). ApoE−/− mice were HFD fed for 4 weeks to induce the development of atherosclerosis and then received IP injections of 4-PBA every 3 days for an additional 4 weeks, during which the mice were continuously HFD fed. In comparison to the mice injected with vehicle control (PBS), those injected with 4-PBA exhibited significantly reduced size of atherosclerotic plaques, as evident from Hematoxylin and Eosin staining (Figure 2A), as well as remarkably diminished lipid deposition in the plaques, as shown by Oil Red O staining (Figure 2B). Moreover, the injection of 4-PBA significantly increased the collagen content within plaques, indicating an improvement in plaque stability (Figure 2C). The plasma levels of total cholesterol, free cholesterol, and triglyceride were also significantly reduced after 4-PBA administration (Figure 2D). Our data validated that long-term IP injection of 4-PBA drastically reduced the atherosclerotic burden in experimental mice.
Figure 2.
Administration of 4-phenylbutyric acid (4-PBA) alleviates atherosclerotic pathogenesis. Male ApoE−/− mice were fed with high-fat diet (HFD) for 4 weeks and intraperitoneally injected with 4-PBA (5 mg/kg body weight) or PBS every 3 days for additional 4 weeks. A, Representative images of H&E-stained atherosclerotic lesions and quantification of plaque size demonstrated as the percentage of lesion area within aortic root area. Scale bars, 300 µm. B, Representative images of oil red O–stained atherosclerotic plaques and quantification of lipid deposition within lesion area. Scale bars, 300 µm. C, Representative images of Picrosirius red–stained atherosclerotic plaques and quantification of collagen content within lesion area. Scale bars, 100 µm. D, Detection of total cholesterol, free cholesterol, and triglyceride levels in the plasma. E through H. Surface expressions of ICAM-1 (intercellular adhesion molecule 1) and CD24 on CD11b+ Ly6G− Ly6Chi monocytes in the peripheral blood (E), bone marrow (BM; F), spleen (G), and aorta (H) were examined by flow cytometry. Data in A through E were analyzed using Student t test, and data in F through H were analyzed using Mann-Whitney U test (n=10 for each group in A through G; n=8 for each group in H; biological replicates). Error bars represent means±SEM.
Consistent with prior independent studies, we also observed proliferating Ly6Chi monocytes in atherosclerotic mice, as evidenced by in vivo Edu incorporation (Figure S4). Notably, administration of 4-PBA substantially increased the frequency of these proliferating monocytes in both the bone marrow and spleen (Figure S4). It is particularly noteworthy that relative to mice receiving vehicle controls, mice injected with 4-PBA exhibited significantly reduced levels of ICAM-1 and elevated levels of CD24 on Ly6Chi and Ly6Clow monocytes harvested from the peripheral blood, bone marrow, and spleen, as well as aorta (Figure 2E through 2H; Figure S5). These data further substantiate that injection of 4-PBA prompts the polarization of monocytes to a resolving state in atherosclerotic mice, which potentially contributes to the amelioration of atherosclerosis pathogenesis.
4-PBA Reduces Monocyte Adhesion by Restoring Pexophagy and Reducing mTOR Signaling
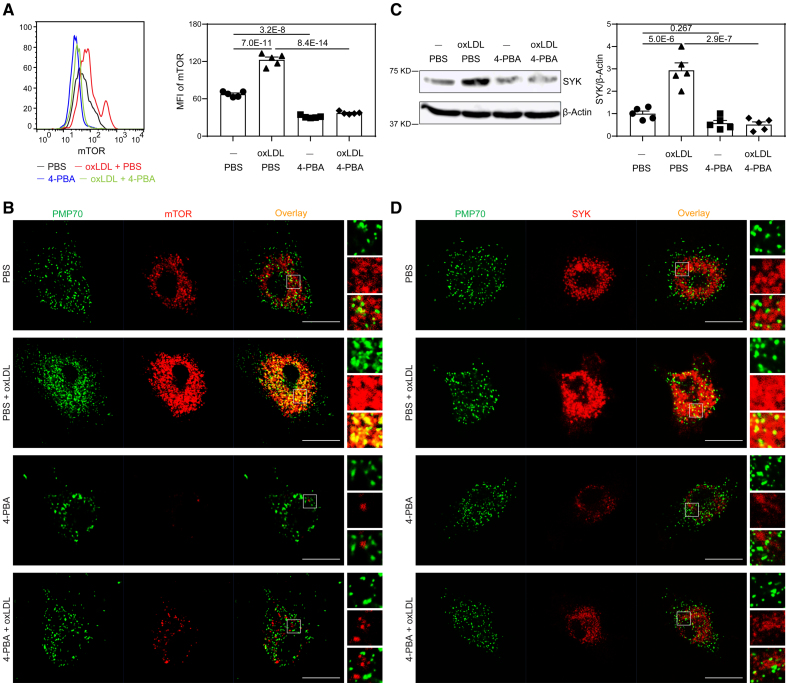
We further examined the molecular and cellular mechanisms responsible for the reprogramming of resolving monocytes by 4-PBA. Previous studies suggest that atherosclerotic stress factors such as oxLDL and/or cholesterol can initiate the inflammatory polarization of monocyte via activation of mTORC1, and that application of mTOR (mammalian target of rapamycin) inhibitor rapamycin effectively reduces atherosclerosis progression.29 mTOR can be activated on the subcellular platforms of lysosomes or peroxisomes by reactive oxygen species (ROS) resulting from defective pexophagy.30,31 Consistent with previous reports, we noted that in contrast to high-dose lipopolysaccharide treatment, oxLDL or low-dose lipopolysaccharide did not compromise mitochondria function, but rather improved mitochondria respiration32 (Figure S6). We, therefore, focused on the effects of oxLDL on inducing the dysfunction of peroxisomes. Flow cytometry analysis revealed a remarkable elevation of intracellular mTOR levels in monocytes after treatment with oxLDL for 5 days (Figure 3A). Intriguingly, we observed that oxLDL drastically increased the subcellular localization of mTOR at PMP70+ peroxisomes (Figure 3B). Moreover, oxLDL also increased cellular levels and peroxisomal distribution of SRC kinase SYK (spleen tyrosine kinase; Figure 3C and 3D), which was shown to form a mutually activating positive feedback loop with mTOR with the support of subcellular ROS.33 These findings corroborate prior studies suggesting that peroxisomes may act as a pivotal platform for sustaining the inflammatory signaling cascade.34
Figure 3.
4-phenylbutyric acid (4-PBA) inhibits mTOR (mammalian target of rapamycin) signaling and restores peroxisome homeostasis in monocytes. Bone marrow–derived monocytes (BMMs) from wild-type (WT) C57 BL/6 mice were cultured in vitro with M-CSF (macrophage colony-stimulating factor; 10 ng/mL) in the presence of oxLDL (oxidized low-density lipoprotein; 10 µg/mL), 4-PBA (1 mmol/L), or PBS for 5 days. A, Representative histogram and quantification of mTOR level in CD11b+ Ly6Chi monocytes as determined by flow cytometry. B, Monocytes were stained with anti-PMP70 and anti-mTOR antibodies, and the localization of PMP70+ peroxisomes and mTOR was examined by confocal microcopy. Scale bars, 10 µm. C, Protein level of SYK in BMMs was examined by Western blotting, and SYK expression was quantified after normalizing to β-Actin expression. D, Monocytes were stained with anti-PMP70 and anti-SYK antibodies, and the localization of peroxisomes and SYK was examined by confocal microcopy. Scale bars, 10 µm. Data in A and C were analyzed using 2-way ANOVA followed by Šídák post hoc test (n=5 for each group; biological replicates). Error bars represent means±SEM.
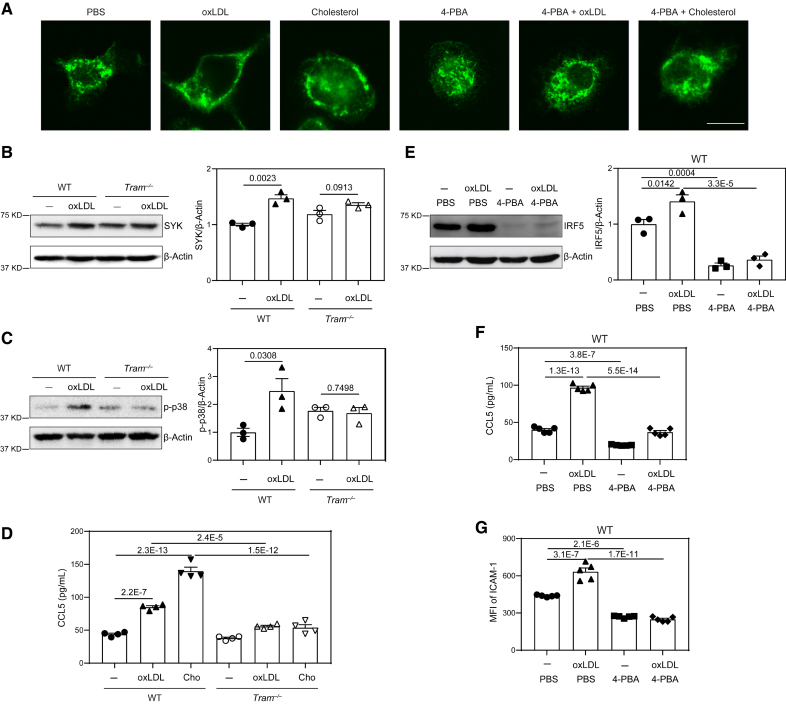
We previously reported that membrane-associated adaptor TRAM (Trif-related adapter molecule; also known as TICAM-2) can transmit the signal of super-low dose lipopolysaccharide and is responsible for causing peroxisomal dysfunction,6,35 as well as inducing key low-grade inflammatory mediators.6,36 Given that oxLDL or free cholesterol can cause generic membrane stress,37–39 and that TRAM is one of the few innate membrane adaptors with lipid anchors to stressed membrane region,40 we next tested the hypothesis that TRAM serves as a general membrane stress sensor capable of mediating the inflammatory effects of oxLDL or free cholesterol in addition to low-dose lipopolysaccharide. As shown in Figure 4A, both oxLDL and free cholesterol promoted TRAM clustering on the cell membrane. We further examined cellular activation in wild-type (WT) and TRAM-deficient monocytes challenged with oxLDL. We found that the potent activation of p38 as well as SYK by oxLDL is observed in WT but not Tram−/− monocytes (Figure 4B and 4C). Functionally, oxLDL or free cholesterol induced ICAM-1 expression and chemokine ligand 5 (CCL5) secretion in WT monocytes but not TRAM-deficient monocytes (Figure 4D). Our data further confirm that oxLDL induces monocyte activation via a generic membrane-associated stress sensor TRAM.
Figure 4.
TRAM (Trif-related adapter molecule) serves as a general membrane stress sensor mediating the inflammatory effects of lipids on monocytes. A, Bone marrow–derived monocytes (BMMs) from wild-type (WT) C57 BL/6 mice were cultured in vitro with M-CSF (macrophage colony-stimulating factor; 10 ng/mL) in the presence of oxLDL (oxidized low-density lipoprotein; 10 µg/mL), cholesterol (10 µg/mL), 4-phenylbutyric acid (4-PBA; 1 mmol/L) or PBS for 5 days. The cells were stained with anti-TRAM antibody, and cellular distribution of TRAM was examined by confocal microcopy. Scale bars, 10 µm. B and C, BMMs from WT C57 BL/6 mice and Tram−/− mice were cultured with M-CSF (10 ng/mL) in the presence of oxLDL (10 µg/mL) or PBS for 5 days. Protein level of SYK (B) and phosphorylation of p-38 (C) were examined by Western blotting and quantified after normalizing to β-Actin expression. D, BMMs from WT C57 BL/6 mice and Tram−/− mice were cultured with M-CSF (10 ng/mL) in the presence of oxLDL (10 µg/mL), cholesterol (10 µg/mL) or PBS for 5 days. Production of chemokine ligand 5 (CCL5) was determined by ELISA. E through G, BMMs from WT C57 BL/6 mice were cultured with M-CSF (10 ng/mL) in the presence of oxLDL (10 µg/mL), 4-PBA (1 mmol/L), or PBS for 5 days. Protein level of IRF5 was examined by Western blotting and quantified after normalizing to β-Actin expression (E). Production of CCL5 was determined by ELISA (F), and surface expression of ICAM-1 on CD11b+ monocytes was determined by flow cytometry (G). Data in B and C were analyzed using Student t test, and data in D through G were analyzed using 2-way ANOVA followed by Šídák post hoc test (n=3 for each group in B, C, and E; n=4 for each group in D; n=5 for each group in F and G; biological replicates. Error bars represent means±SEM.
To further validate that low-dose lipopolysaccharide and cholesterol can similarly reprogram monocyte memory, we then examined genome-wide methylation profiles of monocytes trained by varying dosages of lipopolysaccharide or cholesterol. Indeed, principle component analyses showed that exhausted monocytes caused by prolonged challenges with high-dose lipopolysaccharide clustered into a distinct population in terms of their methylation profile. By contrast, monocytes programed by either low-dose lipopolysaccharide or cholesterol clustered together (Figure S7A). We further performed gene ontology (GO) analyses of gene methylation profiles and validated the resemblance of monocytes trained by low-dose lipopolysaccharide or cholesterol (Figure S7B; Data Set S1). Mechanistically, previous studies reveal the importance of IRF5 (interferon regulatory factor 5) in initiating and sustaining low-grade inflammatory monocyte polarization as well as in accelerating atherosclerosis progression.35,41 While prolonged treatment with higher levels of lipopolysaccharide reduces IRF5 levels and causes endotoxin tolerance, low-dose lipopolysaccharide was shown to sustain and activate IRF5.35 Through targeted pyrosequencing analyses, we observed that the IRF5 enhancer region was significantly methylated in high-dose lipopolysaccharide tolerant monocytes, and remain un-methylated in monocytes trained by either low-dose lipopolysaccharide or cholesterol (Figure S7C). Based on these analyses, we validated the protein levels of IRF5 in trained monocytes. We observed that monocytes trained with oxLDL exhibited significantly elevated levels of IRF5 protein, and in sharp contrast, 4-PBA treatment drastically ablated IRF5 expression (Figure 4E), mechanistically validating the anti-inflammatory effects of 4-PBA on trained monocytes.
Next, we tested whether 4-PBA treatment may diffuse the membrane clustering of TRAM via confocal microscopy. We observed that co-incubation of 4-PBA indeed diffused the membrane clustering of TRAM and caused a permissive cytosolic distribution of TRAM (Figure 4A). At the signaling level, we observed that 4-PBA treatment drastically reduced the cellular levels of SYK and mTOR, as well as the co-localization of mTOR and SYK with peroxisome (Figure 3). Functionally, we observed that 4-PBA significantly reduced the expression of ICAM-1 and CCL5 in oxLDL-treated cells (Figure 4F and 4G). Of note, CCL5 was recently implicated via a comprehensive bioinformatics study as the most relevant inflammatory mediator underlying human atherosclerosis.42 Collectively, our data reveal that 4-PBA can mitigate the proinflammatory memory of monocytes through the attenuation of TRAM-mediated subcellular inflammatory stress.
4-PBA Promotes the Expression of Anti-Inflammatory Mediator CD24 Through Enhanced PPARγ Activation
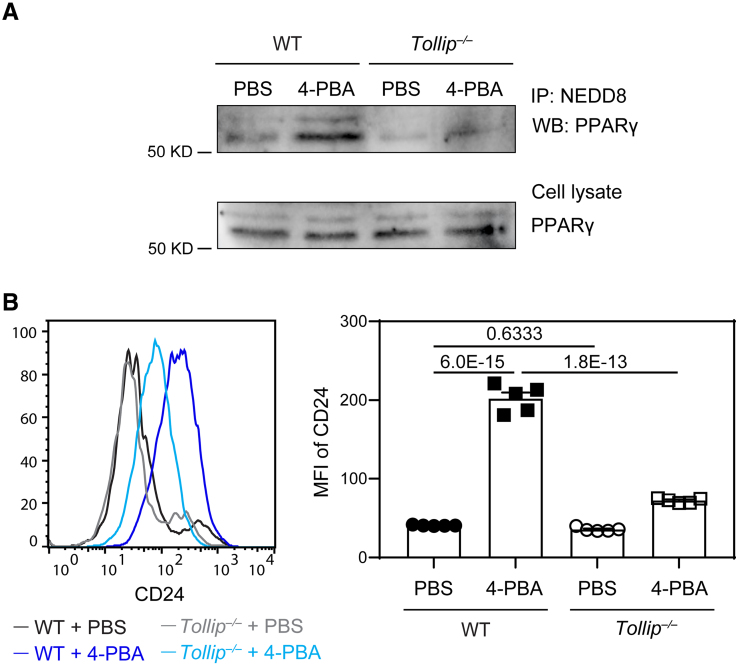
4-PBA not only reduced monocyte inflammation and the expression of adhesion molecule ICAM-1 but also uniformly elevated the expression levels of anti-inflammatory mediator CD24. To further characterize the molecular mechanism for elevated CD24 expression, we examined genes induced by 4-PBA in the single-cell RNA sequencing data set and noted the elevated expression of NEDD8 (neural precursor cell expressed, developmentally downregulated 8; Figure 1A through 1C). NEDD8-mediated PPARγ (peroxisome proliferator-activated receptor γ) neddylation was shown to potently enhance PPARγ activation,43 which may be responsible for the elevated induction of CD24. We tested the status of PPARγ neddylation by co-immunoprecipitation and observed increased PPARγ neddylation in monocytes trained by 4-PBA (Figure 5A).
Figure 5.
4-phenylbutyric acid (4-PBA) promotes the expression of CD24 through enhanced PPARγ (peroxisome proliferator-activated receptor γ) activation in a Tollip-dependent manner. Bone marrow–derived monocytes (BMMs) from wild-type (WT) C57 BL/6 mice and Tollip−/− mice were cultured in vitro with M-CSF (macrophage colony-stimulating factor; 10 ng/mL) in the presence of 4-PBA (1 mmol/L) or PBS for 5 days. A, Cell lysate was isolated and subjected to immunoprecipitation with anti-NEDD8 antibodies conjugated to resin. The association of PPARγ with NEDD8 was examined by Western blotting. PPARγ in cell lysate was also examined by Western blotting. B, Surface expression of CD24 on CD11b+ Ly6G− Ly6Chi monocytes was examined by flow cytometry. Mean Fluorescence Intensity (MFI) of CD24 was quantified. Data in B were analyzed using 2-way ANOVA followed by Šídák post hoc test (n=5 for each group; biological replicates). Error bars represent means±SEM.
To clearly define molecular mechanisms responsible for increased PPARγ neddylation by 4-PBA, we next examined NEDD8-interacting molecule TOLLIP (toll-interacting protein), discovered through an unbiased proteomic analysis of peptides coprecipitated with TOLLIP (Data Set S2). TOLLIP was also independently identified as an important mediator for subcellular organelle fusion and homeostasis.44,45 Furthermore, Tollip deletion results in the expansion of inflammatory monocytes and exacerbates atherosclerosis.46 We tested whether Tollip deficiency ablates the beneficial effects of 4-PBA on monocyte resolution. As shown in Figure 5A, PPARγ neddylation was reduced in Tollip-deficient monocytes. Functionally, we observed that Tollip deletion significantly dampened the induction of CD24 on monocytes by 4-PBA (Figure 5B; Figure S8). Our data indicate that treatment with 4-PBA promotes PPARγ neddylation and CD24 expression in monocytes in a TOLLIP-dependent manner.
CD24 Potentiates Inflammation Resolution by 4-PBA–Trained Monocytes
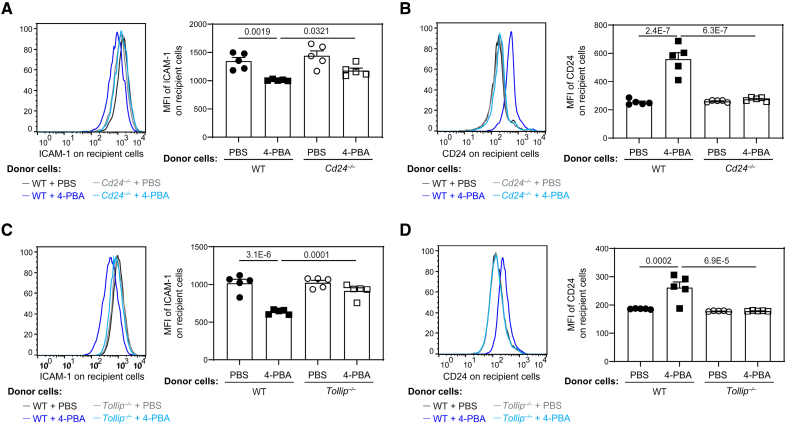
CD24 serves as a potent anti-inflammatory mediator and was independently shown to reduce the inflammatory activation of neighboring cells.47 We next established a coculture system to determine whether 4-PBA programmed monocytes effectively propagate inflammation resolution to neighboring cells through CD24. BMMs from B6.SJL mice (CD45.1+) were pretreated with oxLDL to induce inflammatory polarization and then co-incubated with BMMs from WT B6 or Cd24−/− mice (CD45.2+) that had been treated with PBS or 4-PBA. After coculture for 2 days, the cell surface expression of ICAM-1 and CD24 on CD45.1+ recipient BMMs was examined by flow cytometry. As shown in Figure 6A and 6B, compared with PBS-treated donor monocytes, 4-PBA programmed donor monocytes effectively propagate inflammation resolution to recipient monocytes previously activated by oxLDL as reflected by reduced expression of ICAM-1 and elevated expression of CD24 on recipient monocytes. Importantly, 4-PBA-treated CD24-deficient monocytes failed to impact the expression of ICAM-1 nor CD24 on neighboring recipient monocytes (Figure 6A and 6B), suggesting that CD24 is required for the propagation of inflammation resolution.
Figure 6.
4-phenylbutyric acid (4-PBA) trained monocytes propagate resolving nature to neighboring monocytes through CD24 and Tollip. A and B, Bone marrow–derived monocytes (BMMs) from wild-type (WT) C57 BL/6 mice and Cd24−/− mice, which are both CD45.2+, were cultured in vitro with M-CSF (macrophage colony-stimulating factor; 10 ng/mL) in the presence of 4-PBA (1 mmol/L) or PBS for 5 days. Recipient BMMs prepared from B6 SJL mice, which are CD45.1+, were treated with oxLDL (oxidized low-density lipoprotein; 10 µg/mL) for 3 days and then cocultured in vitro with CD45.2+ donor cells for 2 days. Surface expressions of ICAM-1 (intercellular adhesion molecule 1; A) and CD24 (B) on CD45.1+ recipient BMMs were examined by flow cytometry. C and D, BMMs from WT C57 BL/6 mice and Tollip−/− mice, which are both CD45.2+, were cultured with M-CSF (10 ng/mL) in the presence of 4-PBA (1 mmol/L) or PBS for 5 days. Recipient BMMs prepared from B6 SJL mice, which are CD45.1+, were treated with oxLDL (10 µg/mL) for 3 days and then cocultured with CD45.2+ donor cells in vitro for 2 days. Surface expression of ICAM-1 (C) and CD24 (D) on CD45.1+ recipient BMMs was examined by flow cytometry. Data were analyzed using 2-way ANOVA followed by Šídák post hoc test (n=5 for each group; biological replicates). Error bars represent means±SEM.
Given that we observed TOLLIP is required for PPARγ neddylation and CD24 induction by 4-PBA, we then tested whether Tollip deletion may similarly abolish the inflammatory resolution by 4-PBA. Indeed, neither expression of ICAM-1 nor CD24 on neighboring recipient monocytes was significantly impacted by Tollip-deficient monocytes trained with 4-PBA (Figure 6C and 6D).
4-PBA–Trained Monocytes Effectively Reduce Atherosclerosis Pathogenesis
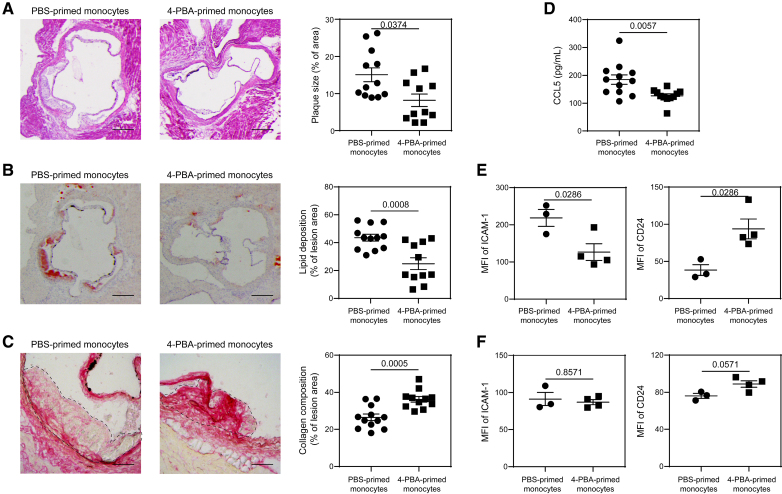
Although systemic administration of 4-PBA can alleviate atherosclerosis progression, it may also interfere with the normal differentiation of monocytes to macrophages, subsequently leading to a decreased number of resident macrophages across various tissues. To enhance the therapeutic specificity of 4-PBA, it is critical to refine its efficacy for targeted purposes. Having defined the phenotypic and mechanistic aspects of resolving monocytes trained by 4-PBA, we then tested whether 4-PBA–trained monocytes are sufficient to render atheroprotection when transfused into recipient experimental animals. Both male and female ApoE−/− mice were subjected to HFD for 4 weeks and then transfused weekly for an additional 4 weeks with BMMs trained with either PBS or 4-PBA. The recipient mice were supplemented with HFD during this process, allowing the development of atherosclerosis. We observed that both male and female recipient mice transfused with 4-PBA–programmed monocytes exhibited a significant reduction in plaque sizes and decreased plaque lipid content (Figure 7A and 7B; Figure S9A and S9B), as well as dramatically higher plaque collagen content relative to mice transfused with control monocytes (Figure 7C; Figure S9C). Moreover, mice transfused with 4-PBA–programmed monocytes had significantly lower plasma levels of CCL5 (Figure 7D).
Figure 7.
Adoptive transfer of monocytes polarized by 4-phenylbutyric acid (4-PBA) alleviates atherosclerosis. A through D, Male ApoE−/− mice, serving as recipients, were fed with high-fat diet (HFD) for 4 weeks. Bone marrow–derived monocytes (BMMs) from ApoE−/− mice were treated with PBS or 4-PBA (1 mmol/L) for 5 days. PBS- or 4-PBA-polarized monocytes (3×106 cells per mouse) were then adoptively transferred by intravenous injection to HFD-fed ApoE−/− mice once a week for 4 weeks. Tissues were harvested 1 week after the last monocyte transfer. A, Representative images of H&E-stained atherosclerotic lesions and quantification of plaque size demonstrated as the percentage of lesion area within aortic root area. Scale bars, 300 µm. B, Representative images of oil red O–stained atherosclerotic plaques and quantification of lipid deposition within lesion area. Scale bars, 300 µm. C, Representative images of Picrosirius red–stained atherosclerotic plaques and quantification of collagen content within lesion area. Scale bars, 100 µm. D, Detection of chemokine ligand 5 (CCL5) level in the plasma by ELISA. E and F, PBS- or 4-PBA-polarized monocytes were labeled with CFSE immediately before adoptive transfer, and tissues were harvested 1 week after the last monocyte transfer. Surface expressions of ICAM-1 and CD24 on host CFSE− CD11b+ Ly6G− Ly6Chi monocytes in the aorta (E) and bone marrow (BM; F) were determined by flow cytometry. Data in A, E, and F were analyzed using Mann-Whitney U test, and data in B through D were analyzed using Student t test (n=12 for PBS-trained monocytes group and n=11 for 4-PBA–trained monocytes group in A through D; n=3 for PBS-trained monocytes group and n=4 for 4-PBA-trained monocytes group in E and F; biological replicates). Error bars represent means±SEM.
Given that our in vitro data demonstrated 4-PBA–trained monocytes effectively propagate anti-inflammatory activity to neighboring monocytes (Figure 6), we hypothesized that injection of 4-PBA-trained monocytes would induce a similar phenotypic change in monocytes in vivo. To test this, we labeled transfused monocytes with carboxyfluorescein succinimidyl ester (CFSE) before injection and then analyzed the surface expression of ICAM-1 and CD24 on CFSE-negative monocytes in recipient mice. We observed elevated CD24 expression on host resident monocytes in the aorta and bone marrow following the adoptive transfer of 4-PBA-trained monocytes when compared with those receiving PBS-trained monocytes (Figure 7E and 7F). Injection of 4-PBA-trained monocytes significantly reduced ICAM-1 expression on host monocytes in the aorta but not in the bone marrow (Figure 7E and 7F), indicating that aortic monocytes of atherosclerotic mice became less adhesive after receiving 4-PBA-programmed monocytes.
We further tested whether transfused monocytes trained by 4-PBA may also propagate their anti-inflammatory effects to other resident immune cells such as neutrophils, B cells, and T cells. We observed that recipient mice transfused with 4-PBA–trained monocytes exhibit anti-inflammatory neutrophils expressing higher levels of CD24 and CD200R (Figure S10A); B regulatory cells expressing higher levels of CD24 (Figure S10B); and CD8 T regulatory cells expressing higher levels of CD122 (Figure S10C), in multiple immune niches such as peripheral blood, bone marrow, spleen in addition to the aortic tissue. CD24hi B cells represent a regulatory B cell subset with potent immunosuppressive capacity.48 CD122+ CD8 T cells are well-characterized T regulatory cells suppressing cytotoxic function of T cells.49 CD200R neutrophil subset was shown to be anti-inflammatory with reduced swarming and elastase release.50 Together, our data suggest that the transfusion of 4-PBA-trained monocytes may propagate inflammation resolution to both innate and adaptive immune niches to propagate their atheroprotective effects.
We performed additional experiments to further validate that the transfused monocytes can indeed infiltrate into the inflamed plaque area and modulate the immune environment of recipient mice. To this regard, we cotransfused an equal amount of PBS-or 4-PBA-trained monocytes labeled by CFSE together with separately cultured and purified Ly6C+ proinflammatory monocyte subsets (independently trained by super-low-dose lipopolysaccharide and purified in vitro) labeled by CellTrace Far Red into atherosclerotic mice to address 2 questions. First, we sought to determine whether the transfused monocytes can effectively traffic into the inflamed aortic plaque area. Second, we aimed to test whether the transfusion of 4-PBA–trained monocytes may influence the aortic infiltration of cotransfused Ly6C+ proinflammatory monocytes (which were well known to potently traffic into the inflamed aortic tissues5,51). Indeed, we observed that 24 hours upon transfusion, CFSE-labeled monocytes trained by either 4-PBA or PBS control can effectively traffic into the aorta (Figure S11A). Transfused CFSE+ monocytes were also detected in the bone marrow (Figure S11B) and spleen (Figure S11C), while a few transfused monocytes migrated to the lymph nodes (Figure S11D). Second, we observed that the numbers of infiltrated Far Red+ proinflammatory monocytes within the aortic tissue were significantly reduced in the group of mice cotransfused with 4-PBA–trained monocytes, as compared with the mice cotransfused with PBS control trained monocytes (Figure S11A). Our data suggest that the aortic infiltration of 4-PBA-trained monocytes may effectively attenuate the recruitment of proinflammatory monocytes to the inflamed plaque area of atherosclerotic mice.
These data collectively highlight the efficacy of the adoptive transfer of 4-PBA–programmed monocytes in mitigating the progression of atherosclerosis. These results also indicate a promising approach to immune cell therapy through utilizing resolving monocytes for the treatment of atherosclerosis.
CD24 Is Required for the Resolving Efficacy of 4-PBA-Trained Monocytes In Vivo
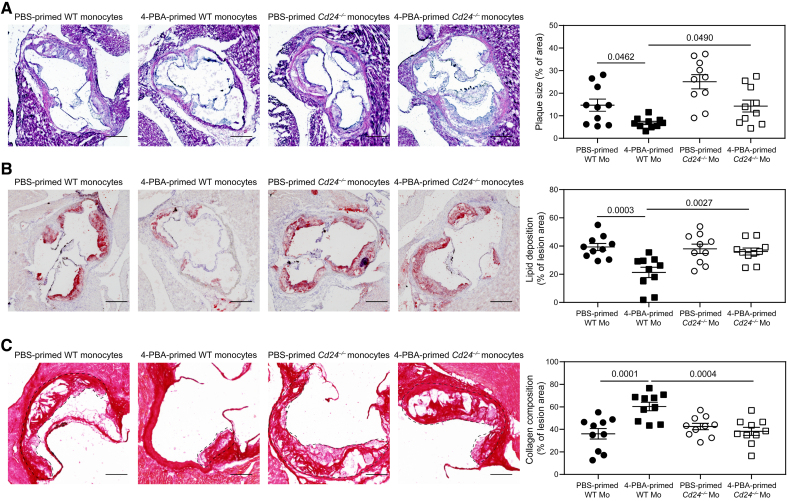
Given the striking effect of 4-PBA training in converting all monocytes into CD24-expressing anti-inflammatory monocytes, we further tested whether the therapeutic effect of 4-PBA–trained monocytes was indeed mediated by CD24. To test this, we used an independent atherosclerosis model with WT C57BL/6 mice intravenously injected with a single dose of AAV8-mPCSK9-D377Y, followed by feeding with HFD for 1 month. Experimental mice were then divided into 4 groups receiving equal amounts of weekly transfusion with the following monocytes: (1) PBS-trained WT monocytes; (2) 4-PBA-trained WT monocytes; (3) PBS-trained Cd24–/– monocytes; and (4) 4-PBA-trained Cd24–/– monocytes, for an additional month on HFD.
We observed that recipient mice transfused with 4-PBA-trained WT monocytes developed significantly reduced atherosclerosis, as compared with recipient mice transfused with PBS-trained control WT monocytes, evidenced by reduced plaque size (Figure 8A); reduced lipid deposition (Figure 8B); and elevated collagen content within the plaques (Figure 8C). In sharp contrast, recipient mice transfused with 4-PBA–trained Cd24–/– monocytes exhibited similar atherosclerosis pathogenesis as compared with mice transfused with PBS-trained Cd24–/– monocytes (Figure 8). Our data suggest that CD24 was indispensable for the resolving efficacy of 4-PBA-trained monocytes, and the induction of CD24 expression and functionality on monocytes by 4-PBA may be crucial for the success of this monocyte-based treatment.
Figure 8.
CD24 mediates the resolving efficacy of 4-phenylbutyric acid (4-PBA)-trained monocytes in atherosclerotic mice. A through C, Male wild-type (WT) C57 BL/6 mice, serving as recipients, were intravenously injected with a single dose of AAV8-mPCSK9-D377Y (5×1011 vector genomes per mouse) and fed with high-fat diet (HFD) for 4 weeks. Bone marrow–derived monocytes (BMMs) from WT C57 BL/6 and Cd24−/− mice were treated with PBS or 4-PBA (1 mmol/L) for 5 days. PBS- or 4-PBA-polarized monocytes (3×106 cells per mouse) were then adoptively transferred by intravenous injection to HFD-fed recipient mice once a week for 4 weeks. Tissues were harvested 1 week after the last monocyte transfer. A, Representative images of H&E-stained atherosclerotic lesions and quantification of plaque size demonstrated as the percentage of lesion area within aortic root area. Scale bars, 300 µm. B, Representative images of Oil Red O–stained atherosclerotic plaques and quantification of lipid deposition within lesion area. Scale bars, 300 µm. C, Representative images of Picrosirius red–stained atherosclerotic plaques and quantification of collagen content within lesion area. Scale bars, 100 µm. Data were analyzed using 2-way ANOVA followed by Šídák post hoc test (n=10 for each group; biological replicates). Error bars represent means±SEM.
DISCUSSION
Our work identified a robust approach for reprogramming resolving monocytes capable of effectively propagating inflammation resolution and treating atherosclerosis. Monocytes arrested by 4-PBA persist in a less-differentiated, proresolving state without further differentiation into the mature and inflammatory macrophages typically involved in exacerbating atherosclerosis. 4-PBA–trained monocytes exhibit reduced levels of adhesion molecule ICAM-1, immune cell chemokine CCL5, and mature macrophage marker F4/80 while also possessing drastically elevated levels of proresolving mediators such as CD24. 4-PBA programmed monocytes can potently propagate inflammation resolution through CD24-mediated intercellular communication with neighboring cells. Mechanistically, we demonstrated that 4-PBA effectively reduces monocyte inflammatory signaling by reducing TRAM-mediated cellular stress, as indicated by reduced peroxisome assembly of mTOR and SYK activation. Concurrently, 4-PBA robustly promotes the expression of anti-inflammatory CD24 by facilitating TOLLIP-mediated PPARγ-neddylation and activation.
Our study outlined a novel immune cell-based therapeutic alternative for the future treatment of atherosclerosis. Previously, 4-PBA was shown to be effective in treating experimental atherosclerosis.19–21 However, systemic administration of chemical compounds such as 4-PBA introduces a plethora of side effects, potentially altering host defense, cellular development, and other vital tissue functions. Our ex vivo characterization demonstrated that monocytes treated with 4-PBA are arrested at a less-mature monocytic state, which suggests that long-term systemic injection of 4-PBA would likely impact systemic macrophage development and host anti-microbial defense. By contrast, the administration of 4-PBA-reprogrammed monocytes would enable clinicians to selectively harness their anti-inflammatory and therapeutic potential while alleviating the side effects of systemic 4-PBA application.
Through integrated single-cell RNA sequencing and relevant functional analyses, our current work not only presents a systematic characterization of 4-PBA programmed resolving monocytes but also reveals key mechanistic insights into monocyte reprogramming dynamics. We determined that 4-PBA reprograms resolving monocytes by reducing peroxisome-mediated mTOR and SYK signaling circuitry. Our findings validate previous reports that mTOR inhibitors such as rapamycin serve as effective agents in reducing atherosclerosis.52 Nanoparticle-mediated delivery of mTOR inhibitors was reported to be effective in treating experimental atherosclerosis.53,54 Our mechanistic studies further defined the TRAM adaptor as a membrane-associated stress sensor of inflamed monocytes and demonstrated that 4-PBA can effectively reduce TRAM-mediated membrane stress. TRAM is one of the few innate signaling adaptors with covalently associated lipid motifs allowing them to be anchored onto the membrane lipid leaflet.55,56 Lipid-modifications such as palmitoylation or myristoylation not only serve to anchor signaling molecules to the cell membrane57–59 but also can facilitate the sensing of membrane stress signals independent of cell surface receptors.60,61 Previous studies revealed that increased membrane stress and/or rigidity can facilitate the assembly of lipid rafts, where lipid-conjugated protein adaptors such as TRAM can dock and undergo activation.62–65 Independent reports demonstrate that cholesterol and oxLDL can generically increase membrane rigidity and lipid raft formation.37–39 Complementing these studies, our current work revealed that oxLDL indeed can cause membrane clustering of the TRAM adaptor in activated monocytes. Our functional data further validated that TRAM may serve as a general membrane stress sensor for monocyte activation given that the induction of ICAM-1 and CCL5 by oxLDL is ablated in TRAM-deficient monocytes. Our data can also reconcile previous independent findings that implicate TRAM as a key signaling adaptor for oxidized phospholipid-induced monocyte activation.66,67 Our data demonstrating the role of 4-PBA in alleviating TRAM clustering and reducing monocyte inflammatory polarization suggest that additional compounds that relieve membrane stress may be similarly effective in dampening monocyte low-grade inflammatory memory.
Our data reveal the key role of CD24 plays in 4-PBA–trained monocytes during the propagation of anti-inflammatory resolution. This is consistent with emerging studies reporting the beneficial roles of CD24 in reducing tissue inflammation.27,68–70 We observed that 4-PBA can potently and uniformly elevate CD24 levels on all monocytes, regardless of their subsets based on the conventional Ly6C levels. Independent studies reveal that CD24 can inhibit inflammatory activation of neighboring cells through ligating its cognate inhibitory receptor Siglec-10.47,68 A recombinant CD24-immunoglobulin protein has been shown to exert anti-inflammatory effects for the treatment of chronic inflammatory diseases such as diabetes.27,68 Our analyses of the aortic local immune environment suggest that 4-PBA–trained monocytes can indeed propagate anti-inflammatory attributes to not only neighboring monocytes and neutrophils but also adaptive T and B cells. The propagation of inflammation resolution may likely be important for the therapeutic efficacy of transfused monocytes. However, our current work only serves as an initial attempt to address the fundamental principle of resolving monocytes in treating atherosclerosis. Future pharmacodynamics and kinetics characterization of trained resolving monocytes are clearly needed to further define their therapeutic potential, which should include their detailed trafficking to various tissues, half-lives, as well as dynamic interactions with systemic and local immune cells. We collected compelling data demonstrating the role of CD24 uniformly elevated on resolving monocytes. However, there are likely other unexplored mechanisms that might also contribute to the resolving phenotype of 4-PBA–trained monocytes. Refinement and purification of monocyte subsets trained by 4-PBA are needed to examine additional mediators involved in propagating homeostasis besides CD24. In addition, 4-PBA–trained monocytes likely would not fully ablate all aspects of atherosclerotic inflammation. Nevertheless, our current study reveals a proof of principle for reprogramming monocytes into a homeostatic resolution state with enhanced cellular expression of CD24, thereby providing a targeted and effective immune cell-based anti-inflammatory therapy capable of treating atherosclerosis and perhaps other chronic inflammatory conditions.
Taken together, our study demonstrates the potential feasibility of 4-PBA–trained monocytes for promoting homeostatic resolution in vitro and in vivo by programming monocytes into a less-differentiated, noninflammatory, and CD24-expressing homeostatic resolving state. We further demonstrate the presence as well as the expansion of CD24-expressing intermediate monocytes by 4-PBA treatment ex vivo, suggesting the feasibility of generating resolving primary human monocytes. Further refinement of innate monocyte-based approaches harbors tremendous promise for generating immune cell-based precision therapies for atherosclerosis.
ARTICLE INFORMATION
Acknowledgments
The authors acknowledge Grace Lee for generating the illustration of the graphic abstract.
Sources of Funding
This study was supported by National Institutes of Health grant HL163948 to L. Li and in part by National Institutes of Health grants R21AG071229, 1R41DK133051-01A1, and R01GM133107-01 to X. Chen.
Disclosures
None.
Supplemental Material
Expanded Materials and Methods
Figures S1–S11
Videos S1 and S2
Data Sets S1–S3
Supplementary Material
Nonstandard Abbreviations and Acronyms
- 4-PBA
- 4-phenylbutyric acid
- BMM
- bone marrow–derived monocyte
- CCL5
- chemokine ligand 5
- CFSE
- carboxyfluorescein succinimidyl ester
- DC
- dendritic cell
- HFD
- high-fat diet
- ICAM-1
- intercellular adhesion molecule 1
- IRF5
- interferon regulatory factor 5
- M-CSF
- macrophage colony–stimulating factor
- mTOR
- mammalian target of rapamycin
- NEDD8
- neural precursor cell expressed, developmentally downregulated 8
- oxLDL
- oxidized low-density lipoprotein
- PPARγ
- peroxisome proliferator-activated receptor γ
- SYK
- spleen tyrosine kinase
- TOLLIP
- toll-interacting protein
- TRAM
- Trif-related adapter molecule
For Sources of Funding and Disclosures, see page 869.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCRESAHA.124.325023.
REFERENCES
- 1.Engelen SE, Robinson AJB, Zurke YX, Monaco C. Therapeutic strategies targeting inflammation and immunity in atherosclerosis: how to proceed? Nat Rev Cardiol. 2022;19:522–542. doi: 10.1038/s41569-021-00668-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soehnlein O, Libby P. Targeting inflammation in atherosclerosis - from experimental insights to the clinic. Nat Rev Drug Discov. 2021;20:589–610. doi: 10.1038/s41573-021-00198-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hetherington I, Totary-Jain H. Anti-atherosclerotic therapies: milestones, challenges, and emerging innovations. Mol Ther. 2022;30:3106–3117. doi: 10.1016/j.ymthe.2022.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010;7:77–86. doi: 10.1038/nrcardio.2009.228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghattas A, Griffiths HR, Devitt A, Lip GY, Shantsila E. Monocytes in coronary artery disease and atherosclerosis: where are we now? J Am Coll Cardiol. 2013;62:1541–1551. doi: 10.1016/j.jacc.2013.07.043 [DOI] [PubMed] [Google Scholar]
- 6.Geng S, Zhang Y, Yi Z, Lu R, Li L. Resolving monocytes generated through TRAM deletion attenuate atherosclerosis. JCI Insight. 2021;6:e149651. doi: 10.1172/jci.insight.149651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tabas I, Lichtman AH. Monocyte-macrophages and T cells in atherosclerosis. Immunity. 2017;47:621–634. doi: 10.1016/j.immuni.2017.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gui Y, Zheng H, Cao RY. Foam cells in atherosclerosis: novel insights into its origins, consequences, and molecular mechanisms. Front Cardiovasc Med. 2022;9:845942. doi: 10.3389/fcvm.2022.845942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saha P, Modarai B, Humphries J, Mattock K, Waltham M, Burnand KG, Smith A. The monocyte/macrophage as a therapeutic target in atherosclerosis. Curr Opin Pharmacol. 2009;9:109–118. doi: 10.1016/j.coph.2008.12.017 [DOI] [PubMed] [Google Scholar]
- 10.Rahman K, Vengrenyuk Y, Ramsey SA, Vila NR, Girgis NM, Liu J, Gusarova V, Gromada J, Weinstock A, Moore KJ, et al. Inflammatory Ly6Chi monocytes and their conversion to M2 macrophages drive atherosclerosis regression. J Clin Invest. 2017;127:2904–2915. doi: 10.1172/JCI75005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kratofil RM, Kubes P, Deniset JF. Monocyte conversion during inflammation and injury. Arterioscler Thromb Vasc Biol. 2017;37:35–42. doi: 10.1161/ATVBAHA.116.308198 [DOI] [PubMed] [Google Scholar]
- 12.Lin JD, Nishi H, Poles J, Niu X, McCauley C, Rahman K, Brown EJ, Yeung ST, Vozhilla N, Weinstock A, et al. Single-cell analysis of fate-mapped macrophages reveals heterogeneity, including stem-like properties, during atherosclerosis progression and regression. JCI Insight. 2019;4:e124574. doi: 10.1172/jci.insight.124574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swirski FK, Hilgendorf I, Robbins CS. From proliferation to proliferation: monocyte lineage comes full circle. Semin Immunopathol. 2014;36:137–148. doi: 10.1007/s00281-013-0409-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yi Z, Geng S, Li L. Comparative analyses of monocyte memory dynamics from mice to humans. Inflamm Res. 2023;72:1539–1549. doi: 10.1007/s00011-023-01762-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee J, Geng S, Li S, Li L. Single cell RNA-Seq and machine learning reveal novel subpopulations in low-grade inflammatory monocytes with unique regulatory circuits. Front Immunol. 2021;12:627036. doi: 10.3389/fimmu.2021.627036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rahtes A, Pradhan K, Sarma M, Xie D, Lu C, Li L. Phenylbutyrate facilitates homeostasis of non-resolving inflammatory macrophages. Innate Immun. 2020;26:62–72. doi: 10.1177/1753425919879503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu N, Qiang W, Kuang X, Thuillier P, Lynn WS, Wong PK. The peroxisome proliferator phenylbutyric acid (PBA) protects astrocytes from ts1 MoMuLV-induced oxidative cell death. J Neurovirol. 2002;8:318–325. doi: 10.1080/13550280290100699 [DOI] [PubMed] [Google Scholar]
- 18.Tanaka H, Okazaki T, Aoyama S, Yokota M, Koike M, Okada Y, Fujiki Y, Gotoh Y. Peroxisomes control mitochondrial dynamics and the mitochondrion-dependent apoptosis pathway. J Cell Sci. 2019;132:jcs224766. doi: 10.1242/jcs.224766 [DOI] [PubMed] [Google Scholar]
- 19.Huang A, Young TL, Dang VT, Shi Y, McAlpine CS, Werstuck GH. 4-phenylbutyrate and valproate treatment attenuates the progression of atherosclerosis and stabilizes existing plaques. Atherosclerosis. 2017;266:103–112. doi: 10.1016/j.atherosclerosis.2017.09.034 [DOI] [PubMed] [Google Scholar]
- 20.Lynn EG, Lhotak S, Lebeau P, Byun JH, Chen J, Platko K, Shi C, O’Brien ER, Austin RC. 4-Phenylbutyrate protects against atherosclerotic lesion growth by increasing the expression of HSP25 in macrophages and in the circulation of Apoe(-/-) mice. FASEB J. 2019;33:8406–8422. doi: 10.1096/fj.201802293RR [DOI] [PubMed] [Google Scholar]
- 21.Erbay E, Babaev VR, Mayers JR, Makowski L, Charles KN, Snitow ME, Fazio S, Wiest MM, Watkins SM, Linton MF, et al. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med. 2009;15:1383–1391. doi: 10.1038/nm.2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asakura E, Hanamura T, Umemura A, Yada K, Yamauchi T, Tanabe T. Effects of macrophage colony-stimulating factor (M-CSF) on lipopolysaccharide (LPS)-induced mediator production from monocytes in vitro. Immunobiology. 1996;195:300–313. doi: 10.1016/S0171-2985(96)80047-7 [DOI] [PubMed] [Google Scholar]
- 23.Pilling D, Fan T, Huang D, Kaul B, Gomer RH. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS One. 2009;4:e7475. doi: 10.1371/journal.pone.0007475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kevil CG, Patel RP, Bullard DC. Essential role of ICAM-1 in mediating monocyte adhesion to aortic endothelial cells. Am J Physiol Cell Physiol. 2001;281:C1442–C1447. doi: 10.1152/ajpcell.2001.281.5.C1442 [DOI] [PubMed] [Google Scholar]
- 25.Patel SS, Thiagarajan R, Willerson JT, Yeh ET. Inhibition of alpha4 integrin and ICAM-1 markedly attenuate macrophage homing to atherosclerotic plaques in ApoE-deficient mice. Circulation. 1998;97:75–81. doi: 10.1161/01.cir.97.1.75 [DOI] [PubMed] [Google Scholar]
- 26.Shakiba N, White CA, Lipsitz YY, Yachie-Kinoshita A, Tonge PD, Hussein SMI, Puri MC, Elbaz J, Morrissey-Scoot J, Li M, et al. CD24 tracks divergent pluripotent states in mouse and human cells. Nat Commun. 2015;6:7329. doi: 10.1038/ncomms8329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Zheng P. CD24-Siglec interactions in inflammatory diseases. Front Immunol. 2023;14:1174789. doi: 10.3389/fimmu.2023.1174789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geng S, Chen K, Yuan R, Peng L, Maitra U, Diao N, Chen C, Zhang Y, Hu Y, Qi CF, et al. The persistence of low-grade inflammatory monocytes contributes to aggravated atherosclerosis. Nat Commun. 2016;7:13436. doi: 10.1038/ncomms13436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y, Yang F, Zou S, Qu L. Rapamycin: a bacteria-derived immunosuppressant that has anti-atherosclerotic effects and its clinical application. Front Pharmacol. 2018;9:1520. doi: 10.3389/fphar.2018.01520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, Kim J, Alexander A, Cai S, Tripathi DN, Dere R, Tee AR, Tait-Mulder J, Di Nardo A, Han JM, et al. A tuberous sclerosis complex signalling node at the peroxisome regulates mTORC1 and autophagy in response to ROS. Nat Cell Biol. 2013;15:1186–1196. doi: 10.1038/ncb2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Betz C, Hall MN. Where is mTOR and what is it doing there? J Cell Biol. 2013;203:563–574. doi: 10.1083/jcb.201306041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pradhan K, Yi Z, Geng S, Li L. Development of exhausted memory monocytes and underlying mechanisms. Front Immunol. 2021;12:778830. doi: 10.3389/fimmu.2021.778830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leseux L, Hamdi SM, Al Saati T, Capilla F, Recher C, Laurent G, Bezombes C. Syk-dependent mTOR activation in follicular lymphoma cells. Blood. 2006;108:4156–4162. doi: 10.1182/blood-2006-05-026203 [DOI] [PubMed] [Google Scholar]
- 34.Dixit E, Boulant S, Zhang Y, Lee AS, Odendall C, Shum B, Hacohen N, Chen ZJ, Whelan SP, Fransen M, et al. Peroxisomes are signaling platforms for antiviral innate immunity. Cell. 2010;141:668–681. doi: 10.1016/j.cell.2010.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan R, Geng S, Li L. Molecular mechanisms that underlie the dynamic adaptation of innate monocyte memory to varying stimulant strength of TLR ligands. Front Immunol. 2016;7:497. doi: 10.3389/fimmu.2016.00497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin R, Zhang Y, Pradhan K, Li L. TICAM2-related pathway mediates neutrophil exhaustion. Sci Rep. 2020;10:14397. doi: 10.1038/s41598-020-71379-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Couto NF, Rezende L, Fernandes-Braga W, Alves AP, Agero U, Alvarez-Leite J, Damasceno NRT, Castro-Gomes T, Andrade LO. OxLDL alterations in endothelial cell membrane dynamics leads to changes in vesicle trafficking and increases cell susceptibility to injury. Biochim Biophys Acta Biomembr. 2020;1862:183139. doi: 10.1016/j.bbamem.2019.183139 [DOI] [PubMed] [Google Scholar]
- 38.Levitan I, Shentu TP. Impact of oxLDL on cholesterol-rich membrane rafts. J Lipids. 2011;2011:730209. doi: 10.1155/2011/730209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chakraborty S, Doktorova M, Molugu TR, Heberle FA, Scott HL, Dzikovski B, Nagao M, Stingaciu LR, Standaert RF, Barrera FN, et al. How cholesterol stiffens unsaturated lipid membranes. Proc Natl Acad Sci USA. 2020;117:21896–21905. doi: 10.1073/pnas.2004807117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barnett KC, Kagan JC. Lipids that directly regulate innate immune signal transduction. Innate Immun. 2020;26:4–14. doi: 10.1177/1753425919852695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leipner J, Dederichs TS, von Ehr A, Rauterberg S, Ehlert C, Merz J, Dufner B, Hoppe N, Krebs K, Heidt T, et al. Myeloid cell-specific Irf5 deficiency stabilizes atherosclerotic plaques in Apoe(-/-) mice. Mol Metab. 2021;53:101250. doi: 10.1016/j.molmet.2021.101250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amadori L, Calcagno C, Fernandez DM, Koplev S, Fernandez N, Kaur R, Mury P, Khan NS, Sajja S, Shamailova R, et al. Systems immunology-based drug repurposing framework to target inflammation in atherosclerosis. Nat Cardiovasc Res. 2023;2:550–571. doi: 10.1038/s44161-023-00278-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park HS, Ju UI, Park JW, Song JY, Shin DH, Lee KH, Jeong LS, Yu J, Lee HW, Cho JY, et al. PPARgamma neddylation essential for adipogenesis is a potential target for treating obesity. Cell Death Differ. 2016;23:1296–1311. doi: 10.1038/cdd.2016.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryan TA, Phillips EO, Collier CL, Jb Robinson A, Routledge D, Wood RE, Assar EA, Tumbarello DA. Tollip coordinates Parkin-dependent trafficking of mitochondrial-derived vesicles. EMBO J. 2020;39:e102539. doi: 10.15252/embj.2019102539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katoh Y, Shiba Y, Mitsuhashi H, Yanagida Y, Takatsu H, Nakayama K. Tollip and Tom1 form a complex and recruit ubiquitin-conjugated proteins onto early endosomes. J Biol Chem. 2004;279:24435–24443. doi: 10.1074/jbc.M400059200 [DOI] [PubMed] [Google Scholar]
- 46.Chen K, Yuan R, Zhang Y, Geng S, Li L. Tollip deficiency alters atherosclerosis and steatosis by disrupting lipophagy. J Am Heart Assoc. 2017;6:e004078. doi: 10.1161/JAHA.116.004078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song NJ, Allen C, Vilgelm AE, Riesenberg BP, Weller KP, Reynolds K, Chakravarthy KB, Kumar A, Khatiwada A, Sun Z, et al. Treatment with soluble CD24 attenuates COVID-19-associated systemic immunopathology. J Hematol Oncol. 2022;15:5. doi: 10.1186/s13045-021-01222-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, Mauri C. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32:129–140. doi: 10.1016/j.immuni.2009.11.009 [DOI] [PubMed] [Google Scholar]
- 49.Akane K, Kojima S, Mak TW, Shiku H, Suzuki H. CD8+CD122+CD49dlow regulatory T cells maintain T-cell homeostasis by killing activated T cells via Fas/FasL-mediated cytotoxicity. Proc Natl Acad Sci USA. 2016;113:2460–2465. doi: 10.1073/pnas.1525098113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin R, Yi Z, Wang J, Geng S, Li L. Generation of resolving memory neutrophils through pharmacological training with 4-PBA or genetic deletion of TRAM. Cell Death Dis. 2022;13:345. doi: 10.1038/s41419-022-04809-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hilgendorf I, Swirski FK, Robbins CS. Monocyte fate in atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:272–279. doi: 10.1161/ATVBAHA.114.303565 [DOI] [PubMed] [Google Scholar]
- 52.Kurdi A, De Meyer GR, Martinet W. Potential therapeutic effects of mTOR inhibition in atherosclerosis. Br J Clin Pharmacol. 2016;82:1267–1279. doi: 10.1111/bcp.12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao W, Zhao Y, Li X, Sun Y, Cai M, Cao W, Liu Z, Tong L, Cui G, Tang B. H(2)O(2)-responsive and plaque-penetrating nanoplatform for mTOR gene silencing with robust anti-atherosclerosis efficacy. Chem Sci. 2018;9:439–445. doi: 10.1039/c7sc03582a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boada C, Zinger A, Tsao C, Zhao P, Martinez JO, Hartman K, Naoi T, Sukhoveshin R, Sushnitha M, Molinaro R, et al. Rapamycin-loaded biomimetic nanoparticles reverse vascular inflammation. Circ Res. 2020;126:25–37. doi: 10.1161/CIRCRESAHA.119.315185 [DOI] [PubMed] [Google Scholar]
- 55.Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol. 2008;9:361–368. doi: 10.1038/ni1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rowe DC, McGettrick AF, Latz E, Monks BG, Gay NJ, Yamamoto M, Akira S, O’Neill LA, Fitzgerald KA, Golenbock DT. The myristoylation of TRIF-related adaptor molecule is essential for Toll-like receptor 4 signal transduction. Proc Natl Acad Sci USA. 2006;103:6299–6304. doi: 10.1073/pnas.0510041103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Resh MD. Covalent lipid modifications of proteins. Curr Biol. 2013;23:R431–R435. doi: 10.1016/j.cub.2013.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Udenwobele DI, Su RC, Good SV, Ball TB, Varma Shrivastav S, Shrivastav A. Myristoylation: an important protein modification in the immune response. Front Immunol. 2017;8:751. doi: 10.3389/fimmu.2017.00751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang B, Dai T, Sun W, Wei Y, Ren J, Zhang L, Zhang M, Zhou F. Protein N-myristoylation: functions and mechanisms in control of innate immunity. Cell Mol Immunol. 2021;18:878–888. doi: 10.1038/s41423-021-00663-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang X, Chatterjee V, Ma Y, Zheng E, Yuan SY. Protein palmitoylation in leukocyte signaling and function. Front Cell Dev Biol. 2020;8:600368. doi: 10.3389/fcell.2020.600368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zareba-Koziol M, Bartkowiak-Kaczmarek A, Figiel I, Krzystyniak A, Wojtowicz T, Bijata M, Wlodarczyk J. Stress-induced changes in the S-palmitoylation and S-nitrosylation of synaptic proteins. Mol Cell Proteomics. 2019;18:1916–1938. doi: 10.1074/mcp.RA119.001581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bolmatov D, Soloviov D, Zhernenkov M, Zav’yalov D, Mamontov E, Suvorov A, Cai YQ, Katsaras J. Molecular picture of the transient nature of lipid rafts. Langmuir. 2020;36:4887–4896. doi: 10.1021/acs.langmuir.0c00125 [DOI] [PubMed] [Google Scholar]
- 63.Zampagni M, Evangelisti E, Cascella R, Liguri G, Becatti M, Pensalfini A, Uberti D, Cenini G, Memo M, Bagnoli S, et al. Lipid rafts are primary mediators of amyloid oxidative attack on plasma membrane. J Mol Med (Berl). 2010;88:597–608. doi: 10.1007/s00109-010-0603-8 [DOI] [PubMed] [Google Scholar]
- 64.Sviridov D, Mukhamedova N, Miller YI. Lipid rafts as a therapeutic target. J Lipid Res. 2020;61:687–695. doi: 10.1194/jlr.TR120000658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang AY, Yi F, Zhang G, Gulbins E, Li PL. Lipid raft clustering and redox signaling platform formation in coronary arterial endothelial cells. Hypertension. 2006;47:74–80. doi: 10.1161/10.1161/01.HYP.0000196727.53300.62 [DOI] [PubMed] [Google Scholar]
- 66.Rao X, Zhao S, Braunstein Z, Mao H, Razavi M, Duan L, Wei Y, Toomey AC, Rajagopalan S, Zhong J. Oxidized LDL upregulates macrophage DPP4 expression via TLR4/TRIF/CD36 pathways. EBioMedicine. 2019;41:50–61. doi: 10.1016/j.ebiom.2019.01.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu Y, Ye J, Guo R, Liang X, Yang L. TRIF regulates BIC/miR-155 via the ERK signaling pathway to control the ox-LDL-induced macrophage inflammatory response. J Immunol Res. 2018;2018:6249085. doi: 10.1155/2018/6249085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang X, Liu M, Zhang J, Brown NK, Zhang P, Zhang Y, Liu H, Du X, Wu W, Devenport M, et al. CD24-Siglec axis is an innate immune checkpoint against metaflammation and metabolic disorder. Cell Metab. 2022;34:1088–1103.e6. doi: 10.1016/j.cmet.2022.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tian RR, Zhang MX, Zhang LT, Zhang P, Ma JP, Liu M, Devenport M, Zheng P, Zhang XL, Lian XD, et al. CD24 and Fc fusion protein protects SIVmac239-infected Chinese rhesus macaque against progression to AIDS. Antiviral Res. 2018;157:9–17. doi: 10.1016/j.antiviral.2018.07.004 [DOI] [PubMed] [Google Scholar]
- 70.Chen GY, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323:1722–1725. doi: 10.1126/science.1168988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Geng S, Zhang Y, Lee C, Li L. Novel reprogramming of neutrophils modulates inflammation resolution during atherosclerosis. Sci Adv. 2019;5:eaav2309. doi: 10.1126/sciadv.aav2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qiao L, Ma J, Zhang Z, Sui W, Zhai C, Xu D, Wang Z, Lu H, Zhang M, Zhang C, et al. Deficient chaperone-mediated autophagy promotes inflammation and atherosclerosis. Circ Res. 2021;129:1141–1157. doi: 10.1161/CIRCRESAHA.121.318908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park K, Li Q, Lynes MD, Yokomizo H, Maddaloni E, Shinjo T, St-Louis R, Li Q, Katagiri S, Fu J, et al. Endothelial cells induced progenitors into brown fat to reduce atherosclerosis. Circ Res. 2022;131:168–183. doi: 10.1161/CIRCRESAHA.121.319582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang J, Wu Y, Lin R, Zhang Y, Li L. TRAM deletion attenuates monocyte exhaustion and alleviates sepsis severity. Front Immunol. 2023;14:1297329. doi: 10.3389/fimmu.2023.1297329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Flynn MC, Kraakman MJ, Tikellis C, Lee MKS, Hanssen NMJ, Kammoun HL, Pickering RJ, Dragoljevic D, Al-Sharea A, Barrett TJ, et al. Transient intermittent hyperglycemia accelerates atherosclerosis by promoting myelopoiesis. Circ Res. 2020;127:877–892. doi: 10.1161/CIRCRESAHA.120.316653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liang G, Wang S, Shao J, Jin YJ, Xu L, Yan Y, Gunther S, Wang L, Offermanns S. Tenascin-X mediates flow-induced suppression of EndMT and atherosclerosis. Circ Res. 2022;130:1647–1659. doi: 10.1161/CIRCRESAHA.121.320694 [DOI] [PubMed] [Google Scholar]
- 77.Li Q, Park K, Xia Y, Matsumoto M, Qi W, Fu J, Yokomizo H, Khamaisi M, Wang X, Rask-Madsen C, et al. Regulation of macrophage apoptosis and atherosclerosis by lipid-induced PKCdelta isoform activation. Circ Res. 2017;121:1153–1167. doi: 10.1161/CIRCRESAHA.117.311606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.De Jesus A, Pusec CM, Nguyen T, Keyhani-Nejad F, Gao P, Weinberg SE, Ardehali H. Optimized protocol to isolate primary mouse peritoneal macrophage metabolites. STAR Protoc. 2022;3:101668. doi: 10.1016/j.xpro.2022.101668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bjorklund MM, Hollensen AK, Hagensen MK, Dagnaes-Hansen F, Christoffersen C, Mikkelsen JG, Bentzon JF. Induction of atherosclerosis in mice and hamsters without germline genetic engineering. Circ Res. 2014;114:1684–1689. doi: 10.1161/CIRCRESAHA.114.302937 [DOI] [PubMed] [Google Scholar]
- 81.El Khoury J, Hickman SE, Thomas CA, Cao L, Silverstein SC, Loike JD. Scavenger receptor-mediated adhesion of microglia to beta-amyloid fibrils. Nature. 1996;382:716–719. doi: 10.1038/382716a0 [DOI] [PubMed] [Google Scholar]
- 82.Sheets K, Wang J, Zhao W, Kapania R, Nain AS. Nanonet force microscopy for measuring cell forces. Biophys J. 2016;111:197–207. doi: 10.1016/j.bpj.2016.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Caldwell BA, Wu Y, Wang J, Li L. Altered DNA methylation underlies monocyte dysregulation and immune exhaustion memory in sepsis. Cell Rep. 2024;43:113894. doi: 10.1016/j.celrep.2024.113894 [DOI] [PubMed] [Google Scholar]
- 84.Zhou W, Triche TJ, Jr, Laird PW, Shen H. SeSAMe: reducing artifactual detection of DNA methylation by Infinium BeadChips in genomic deletions. Nucleic Acids Res. 2018;46:e123. doi: 10.1093/nar/gky691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mori M, Sakamoto A, Kawakami R, Guo L, Slenders L, Mosquera JV, Ghosh SKB, Wesseling M, Shiraki T, Bellissard A, et al. CD163(+) macrophages induce endothelial-to-mesenchymal transition in atheroma. Circ Res. 2024;135:e4–e23. doi: 10.1161/CIRCRESAHA.123.324082 [DOI] [PubMed] [Google Scholar]
- 86.Krishnan J, Hennen EM, Ao M, Kirabo A, Ahmad T, de la Visitacion N, Patrick DM. NETosis drives blood pressure elevation and vascular dysfunction in hypertension. Circ Res. 2024;134:1483–1494. doi: 10.1161/CIRCRESAHA.123.323897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li S, He RC, Wu SG, Song Y, Zhang KL, Tang ML, Bei YR, Zhang T, Lu JB, Ma X, et al. LncRNA PSMB8-AS1 instigates vascular inflammation to aggravate atherosclerosis. Circ Res. 2024;134:60–80. doi: 10.1161/CIRCRESAHA.122.322360 [DOI] [PubMed] [Google Scholar]
- 88.Ma W, Jia K, Cheng H, Xu H, Li Z, Zhang H, Xie H, Sun H, Yi L, Chen Z, et al. Orphan nuclear receptor NR4A3 promotes vascular calcification via histone lactylation. Circ Res. 2024;134:1427–1447. doi: 10.1161/CIRCRESAHA.123.323699 [DOI] [PubMed] [Google Scholar]
- 89.Mendoza A, Patel P, Robichaux D, Ramirez D, Karch J. Inhibition of the mPTP and lipid peroxidation is additively protective against I/R injury. Circ Res. 2024;134:1292–1305. doi: 10.1161/CIRCRESAHA.123.323882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bao Q, Zhang B, Zhou L, Yang Q, Mu X, Liu X, Zhang S, Yuan M, Zhang Y, Che J, et al. CNP ameliorates macrophage inflammatory response and atherosclerosis. Circ Res. 2024;134:e72–e91. doi: 10.1161/CIRCRESAHA.123.324086 [DOI] [PubMed] [Google Scholar]
- 91.Lv JJ, Wang H, Zhang C, Zhang TJ, Wei HL, Liu ZK, Ma YH, Yang Z, He Q, Wang LJ, et al. CD147 sparks atherosclerosis by driving M1 phenotype and impairing efferocytosis. Circ Res. 2024;134:165–185. doi: 10.1161/CIRCRESAHA.123.323223 [DOI] [PubMed] [Google Scholar]
- 92.Ye S, Huang H, Han X, Luo W, Wu L, Ye Y, Gong Y, Zhao X, Huang W, Wang Y, et al. Dectin-1 acts as a non-classical receptor of Ang II to induce cardiac remodeling. Circ Res. 2023;132:707–722. doi: 10.1161/CIRCRESAHA.122.322259 [DOI] [PubMed] [Google Scholar]
- 93.Chakraborty A, Li Y, Zhang C, Li Y, Rebello KR, Li S, Xu S, Vasquez HG, Zhang L, Luo W, et al. Epigenetic induction of smooth muscle cell phenotypic alterations in aortic aneurysms and dissections. Circulation. 2023;148:959–977. doi: 10.1161/CIRCULATIONAHA.123.063332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Banerjee R, Knauer LA, Iyer D, Barlow SE, Shalaby H, Dehghan R, Scallan JP, Yang Y. Rictor, an mTORC2 protein, regulates murine lymphatic valve formation through the AKT-FOXO1 signaling. Arterioscler Thromb Vasc Biol. 2024;44:2004–2023. doi: 10.1161/ATVBAHA.124.321164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Antonelli LR, Leoratti FM, Costa PA, Rocha BC, Diniz SQ, Tada MS, Pereira DB, Teixeira-Carvalho A, Golenbock DT, Goncalves R, et al. The CD14+CD16+ inflammatory monocyte subset displays increased mitochondrial activity and effector function during acute Plasmodium vivax malaria. PLoS Pathog. 2014;10:e1004393. doi: 10.1371/journal.ppat.1004393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun F, Cheng Y, Wanchai V, Guo W, Mery D, Xu H, Gai D, Siegel E, Bailey C, Ashby C, et al. Bispecific BCMA/CD24 CAR-T cells control multiple myeloma growth. Nat Commun. 2024;15:615. doi: 10.1038/s41467-024-44873-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Khanna A, Bhushan B, Chauhan PS, Saxena S, Gupta DK, Siraj F. High mTOR expression independently prognosticates poor clinical outcome to induction chemotherapy in acute lymphoblastic leukemia. Clin Exp Med. 2018;18:221–227. doi: 10.1007/s10238-017-0478-x [DOI] [PubMed] [Google Scholar]
- 98.Keller B, Stumpf I, Strohmeier V, Usadel S, Verhoeyen E, Eibel H, Warnatz K. High SYK expression drives constitutive activation of CD21(low) B cells. J Immunol. 2017;198:4285–4292. doi: 10.4049/jimmunol.1700079 [DOI] [PubMed] [Google Scholar]
- 99.Guiteras J, Ripoll E, Bolanos N, De Ramon L, Fontova P, Lloberas N, Cruzado JM, Aran JM, Avino A, Eritja R, et al. The gene silencing of IRF5 and BLYSS effectively modulates the outcome of experimental lupus nephritis. Mol Ther Nucleic Acids. 2021;24:807–821. doi: 10.1016/j.omtn.2021.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Calvo-Alvarez E, D’Alessandro S, Zanotta N, Basilico N, Parapini S, Signorini L, Perego F, Maina KK, Ferrante P, Modenese A, et al. Multiplex array analysis of circulating cytokines and chemokines in COVID-19 patients during the first wave of the SARS-CoV-2 pandemic in Milan, Italy. BMC Immunol. 2024;25:49. doi: 10.1186/s12865-024-00641-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kinstrie R, Horne GA, Morrison H, Irvine D, Munje C, Castaneda EG, Moka HA, Dunn K, Cassels JE, Parry N, et al. CD93 is expressed on chronic myeloid leukemia stem cells and identifies a quiescent population which persists after tyrosine kinase inhibitor therapy. Leukemia. 2020;34:1613–1625. doi: 10.1038/s41375-019-0684-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shi D, Che J, Yan Y, Peng B, Yao X, Guo C. Expression and clinical value of CD105 in renal cell carcinoma based on data mining in The Cancer Genome Atlas. Exp Ther Med. 2019;17:4499–4505. doi: 10.3892/etm.2019.7493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Maaninka K, Neuvonen M, Kerkela E, Hyvarinen K, Palviainen M, Kamali-Moghaddam M, Federico A, Greco D, Laitinen S, Oorni K, et al. OxLDL sensitizes platelets for increased formation of extracellular vesicles capable of finetuning macrophage gene expression. Eur J Cell Biol. 2023;102:151311. doi: 10.1016/j.ejcb.2023.151311 [DOI] [PubMed] [Google Scholar]
- 104.Bernardo D, Marin AC, Fernandez-Tome S, Montalban-Arques A, Carrasco A, Tristan E, Ortega-Moreno L, Mora-Gutierrez I, Diaz-Guerra A, Caminero-Fernandez R, et al. Human intestinal pro-inflammatory CD11c(high)CCR2(+)CX3CR1(+) macrophages, but not their tolerogenic CD11c(-)CCR2(-)CX3CR1(-) counterparts, are expanded in inflammatory bowel disease. Mucosal Immunol. 2018;11:1114–1126. doi: 10.1038/s41385-018-0030-7 [DOI] [PubMed] [Google Scholar]
- 105.Peckert-Maier K, Langguth P, Strack A, Stich L, Muhl-Zurbes P, Kuhnt C, Drassner C, Zinser E, Wrage M, Mattner J, et al. CD83 expressed by macrophages is an important immune checkpoint molecule for the resolution of inflammation. Front Immunol. 2023;14:1085742. doi: 10.3389/fimmu.2023.1085742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rolig AS, Rose DC, McGee GH, Rubas W, Kivimae S, Redmond WL. Combining bempegaldesleukin (CD122-preferential IL-2 pathway agonist) and NKTR-262 (TLR7/8 agonist) improves systemic antitumor CD8(+) T cell cytotoxicity over BEMPEG+RT. J ImmunoTher Cancer. 2022;10:e004218. doi: 10.1136/jitc-2021-004218 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All supporting data of this study are available from the corresponding author upon reasonable request. RNA-seq data have been uploaded to the NCBI Gene Expression Omnibus and are accessible under accession number GSE160450. Methylation profiling data have been uploaded to the NCBI Gene Expression Omnibus and are accessible under accession number GSE243358. For detailed experimental methods, materials, and statistical analysis, please see the Supplemental Material and the Major Resource Table.