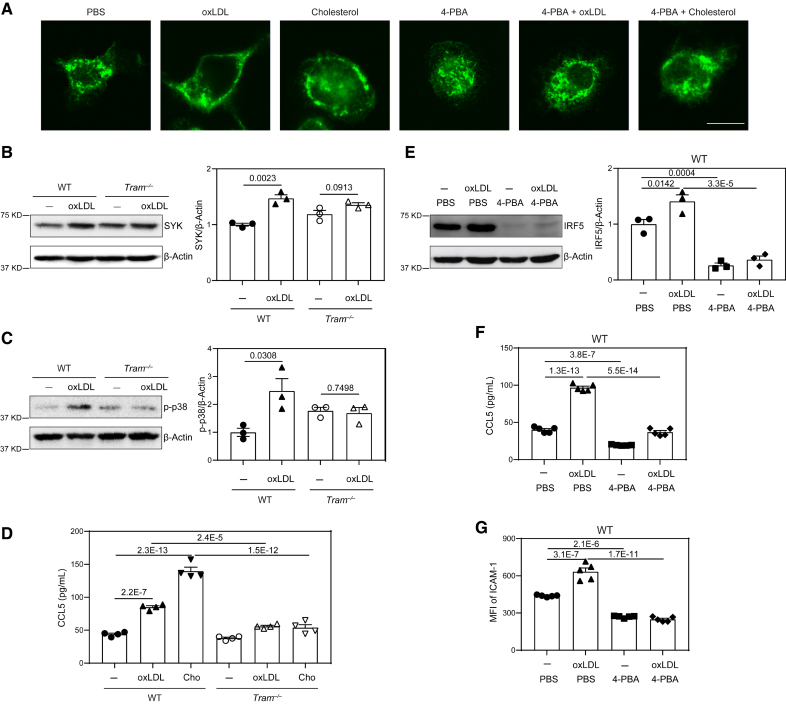
Figure 4.
TRAM (Trif-related adapter molecule) serves as a general membrane stress sensor mediating the inflammatory effects of lipids on monocytes. A, Bone marrow–derived monocytes (BMMs) from wild-type (WT) C57 BL/6 mice were cultured in vitro with M-CSF (macrophage colony-stimulating factor; 10 ng/mL) in the presence of oxLDL (oxidized low-density lipoprotein; 10 µg/mL), cholesterol (10 µg/mL), 4-phenylbutyric acid (4-PBA; 1 mmol/L) or PBS for 5 days. The cells were stained with anti-TRAM antibody, and cellular distribution of TRAM was examined by confocal microcopy. Scale bars, 10 µm. B and C, BMMs from WT C57 BL/6 mice and Tram−/− mice were cultured with M-CSF (10 ng/mL) in the presence of oxLDL (10 µg/mL) or PBS for 5 days. Protein level of SYK (B) and phosphorylation of p-38 (C) were examined by Western blotting and quantified after normalizing to β-Actin expression. D, BMMs from WT C57 BL/6 mice and Tram−/− mice were cultured with M-CSF (10 ng/mL) in the presence of oxLDL (10 µg/mL), cholesterol (10 µg/mL) or PBS for 5 days. Production of chemokine ligand 5 (CCL5) was determined by ELISA. E through G, BMMs from WT C57 BL/6 mice were cultured with M-CSF (10 ng/mL) in the presence of oxLDL (10 µg/mL), 4-PBA (1 mmol/L), or PBS for 5 days. Protein level of IRF5 was examined by Western blotting and quantified after normalizing to β-Actin expression (E). Production of CCL5 was determined by ELISA (F), and surface expression of ICAM-1 on CD11b+ monocytes was determined by flow cytometry (G). Data in B and C were analyzed using Student t test, and data in D through G were analyzed using 2-way ANOVA followed by Šídák post hoc test (n=3 for each group in B, C, and E; n=4 for each group in D; n=5 for each group in F and G; biological replicates. Error bars represent means±SEM.