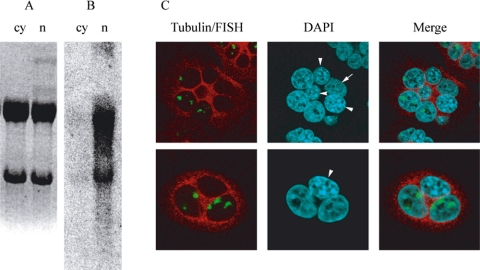
Figure 1.
Subcellular localization of SatIII transcripts. For Northern analysis, HeLa cells were heat shocked 1 h at 42°C and allowed to recover at 37°C for 3 h before harvesting. Nuclear (n) and cytoplasmic (cy) RNA was purified as described in Materials and Methods. The RNA was electrophoresed on an agarose gel and stained with ethidium bromide (A). The RNA was then transferred to a nylon membrane and analyzed in Northern blotting with a probe recognizing SatIII sequences (pHuR98) (B). To assess for nucleocytoplasmic shuttling, an interspecies heterokaryon assay was performed. HeLa and NIH/3T3 cells were grown together on coverslips and fused for 2 min with PEG-4000. Two hours later, the cells were incubated at 42°C for 1 h and then allowed to recover for 2 h at 37°C before fixing. Cells were then stained with RNA-FISH by using the reverse oligo, and with anti-tubulin antibody, and counterstained with DAPI to distinguish between the HeLa cells that stain diffusely and NIH/3T3 that stain with a distinct speckled pattern. Arrow, HeLa cell without nSBs. Arrowheads, mouse nuclei.