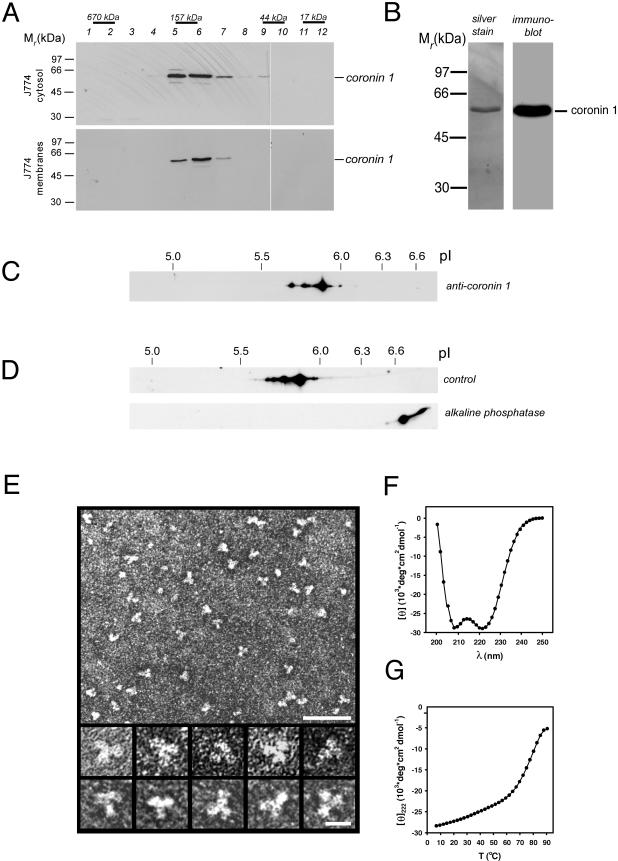
Figure 3.
Molecular organization of the coronin 1 complex. (A) Isolated cytosol (top) and membranes (bottom) from mouse macrophages were solubilized in 2% octyl glucopyranoside and subjected to size exclusion chromatography on a Superdex 200 column. The eluted fractions were assayed for the presence of coronin 1 by SDS-PAGE and immunoblotting. The positions of proteins of known Mr are indicated. (B) Silver-stained SDS-PAGE (left) and immunoblot (right) of affinity-purified coronin 1 molecules from macrophage cytosol by using anti-coronin antiserum. (C) Detection of different coronin 1 isoforms in macrophage lysates by two-dimensional IEF/SDS-PAGE and immunoblotting by using anti-coronin 1 antibodies. (D) Analysis of control and ALP-treated affinity-purified coronin 1. J774 cells were metabolically labeled for 16 h with [35S]methionine, lysed in Triton X-100 lysis buffer, and coronin 1 complexes were purified from the cell lysate by immunoprecipitation by using an anti-coronin 1 antibody. The purified coronin 1 complexes were incubated at 37°C for 2 h with ALP or buffer alone, before analysis by two-dimensional IEF/SDS-PAGE and fluorography. (E) Transmission electron micrographs of affinity-purified and negatively stained coronin 1 complexes. Top, low-magnification overview. Bar, 50 nm. Bottom, high-magnification gallery. Bar, 10 nm. (F and G) CD analysis of ccCor1. Far-UV CD spectra (F) and thermal unfolding recorded by CD at 222 nm (G) were performed in 5 mM sodium phosphate, pH 7.4, containing 150 mM NaCl and at a peptide concentration of 0.15 mg/ml.