Abstract
Aims
Congestive heart failure (HF) is a common complication in patients with acute myocardial infarction (AMI). The estimated plasma volume status [ePVS = (100 − haematocrit)/haemoglobin] is used as the blood plasma volume index to determine the presence of congestion in patients with HF. However, the clinical impact of ePVS at discharge in patients with AMI remains unclear. This study aimed to investigate whether ePVS at discharge could determine the long‐term prognosis in patients with AMI.
Methods and results
We retrospectively identified patients with AMI with ePVS measured at discharge between January 2012 and December 2020. The primary endpoint was post‐discharge all‐cause death. The patients were divided into two groups according to an ePVS cut‐off value of 5.5%, which is commonly used in HF. In total, 1012 patients with AMI were included. The median age was 70 years (range, 61–78 years), and 76.4% of the patients were male. The ePVS > 5.5% (high‐ePVS) group included 365 patients (36.1%), and the all‐cause mortality rate in the total cohort was 17.7%. The log‐rank test revealed that the high‐ePVS group had a significantly higher rate of all‐cause death than the ePVS ≤ 5.5% (low‐ePVS) group (P < 0.001). Multivariate Cox proportional hazards model analysis revealed that high ePVS was associated with post‐discharge all‐cause death, independent of other risk factors (hazard ratio = 1.879; 95% confidence interval = 1.343–2.629, P < 0.001).
Conclusions
High ePVS at discharge was independently associated with high post‐discharge all‐cause mortality in patients with AMI. Our study suggests that ePVS at discharge in patients with AMI could serve as a novel prognostic marker.
Keywords: Myocardial infarction, Estimated plasma volume status, Heart failure, Congestion
Introduction
Among patients with acute myocardial infarction (AMI), congestive heart failure (HF) is a major complication and the most influential predictor of death. 1 Moreover, residual congestion at discharge in patients with AMI is independently associated with an increased risk of all‐cause mortality and readmissions for HF. 2 , 3 , 4 Therefore, targeting congestion at discharge is important to improve the prognosis in patients with AMI. Congestion leads to decreased coronary blood flow and reduced organ return. 5 Therefore, it is important to properly assess congestion. However, this is difficult because currently, there is no precise, non‐invasive method of measurement.
The estimated plasma volume status (ePVS), which is derived from the haemoglobin (Hb) and haematocrit (Hct) values (Strauss formula), is used as the blood plasma volume (PV) index and shows a good correlation with the measured PV. 6 Previous studies have shown that high ePVS is associated with poor prognoses and have proposed a threshold of >5.5 mL/g (high ePVS) as an indicator of excessive congestion in patients with acute decompensated HF (ADHF). 6 , 7
Thus, ePVS can be used to assess congestion at discharge in patients with AMI; however, its impact is yet to be reported. The present study aimed to investigate whether ePVS at discharge could determine the long‐term prognosis of patients with AMI.
Methods
Study design and patients
The Nara Registry and Analysis for Myocardial Infarction Study (NARA‐MI Study) is a dynamic cohort study comprising 1095 patients with AMI who underwent emergency percutaneous coronary intervention (PCI) at Nara Medical University Hospital between January 2012 and December 2020. The diagnosis of AMI included ST‐elevation myocardial infarction (STEMI) and non‐STEMI (NSTEMI) within 48 h of AMI onset. STEMI was defined as persistent symptoms of myocardial ischaemia, ST‐segment elevation at the J‐point in two contiguous leads or a new left bundle branch block on 12‐lead electrocardiography, and high cardiac marker levels [creatine kinase (CK)‐myocardial band or troponin]. NSTEMI was defined as persistent symptoms of myocardial ischaemia in the absence of ST‐segment elevation on electrocardiography, with elevated cardiac marker levels. 8
Primary PCI was performed using standard techniques and catheters via the femoral or radial approach, according to the operator's usual practice. We divided the patients with AMI into two groups according to the ePVS (>5.5%, ≤5.5%) values at discharge (Figure 1). We investigated the impact of ePVS values on AMI prognosis. The Ethics Committee of Nara Medical University approved the study protocol (approval number 1759‐2). Written informed consent was obtained from all patients; this investigation conforms with the principles outlined in the Declaration of Helsinki.
Figure 1.
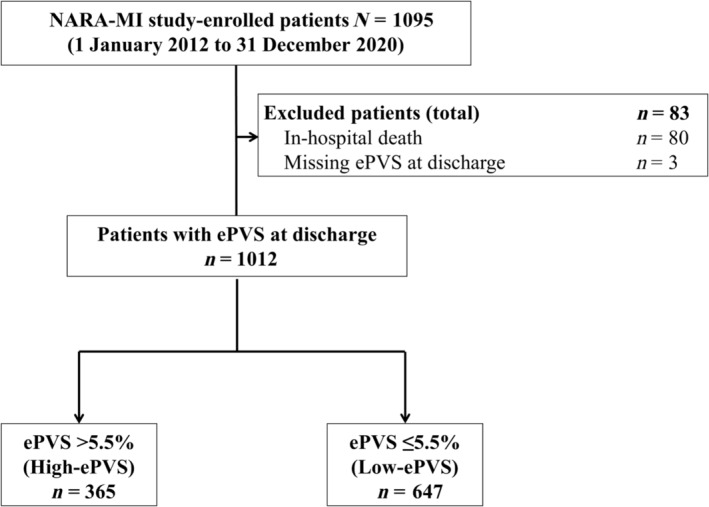
Flow chart of the study cohort. ePVS, estimated plasma volume status; NARA‐MI study, Nara Registry and Analysis for Myocardial Infarction study.
Data collection and definitions
Laboratory parameters, including Hb, Hct, albumin, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, triglyceride, blood urea nitrogen, creatinine, estimated glomerular filtration rate (eGFR) according to the dietary modifications of renal disease method, serum electrolytes (sodium and potassium), CK, and B‐type natriuretic peptide (BNP) levels, were measured in all patients at discharge. Vital signs, including heart rate and blood pressure, at discharge were recorded.
ePVS was calculated using the Strauss‐derived Duarte formula with Hct and Hb values 6 , 9 :
For loop diuretics other than furosemide, the dose was converted into furosemide equivalent doses: 4 mg of torasemide and 30 mg of azosemide were both considered to be equal to 20 mg of furosemide. 10 , 11
Outcomes
The primary endpoint was post‐discharge all‐cause death in time‐to‐event analysis. The secondary endpoints were (1) post‐discharge cardiovascular death and (2) readmission for HF in time‐to‐event analysis. We also conducted a comparative analysis of echocardiographic data [left ventricular ejection fraction (LVEF), left ventricular end‐diastolic volume index (LVEDVI), and left ventricular end‐systolic volume index (LVESVI)] at the time of discharge and 1 year after discharge. The status of all patients was surveyed, and information on outcomes was obtained from the patient medical records and participating cardiologists. When this information was unavailable in the medical records, the clinicians sent letters to the patient homes or telephoned them or their families to request data.
Statistical analysis
Continuous variables were expressed as means and standard deviations for normally distributed variables and as medians with interquartile ranges for non‐normally distributed variables. The Kolmogorov–Smirnov test was used to assess normality. Categorical data were expressed as numbers and percentages. The difference between the two groups was tested using the Student's t‐test for normally distributed variables and the Mann–Whitney U test for non‐normally distributed variables. The χ 2 test was used to compare categorical variables.
To evaluate the association between ePVS values at discharge and outcomes, Kaplan–Meier analyses with log‐rank tests and univariate and multivariate Cox proportional hazards analyses were performed. We selected the ePVS value (5.5%) that is commonly used as an indicator of excessive congestion in patients with ADHF. 6 In the multivariate analysis, the following variables were selected as pre‐existing and known prognostic factors for AMI: age, sex, Killip classification III or IV, hypertension, dyslipidaemia, diabetes mellitus (DM), smoking, atrial fibrillation, peripheral artery disease, history of MI, history of stroke, multivessel disease, LVEF at discharge, eGFR, angiotensin‐converting enzyme inhibitors (ACEis) or angiotensin receptor blockers (ARBs) or angiotensin receptor neprilysin inhibitors (ARNIs), beta‐blockers, aldosterone antagonists, and statins at discharge. 12 , 13 Prior to conducting multivariate analysis, a thorough check for missing data was performed. The dataset used in this study was found to be complete, without any missing values across all variables. In addition to the Cox proportional hazards analysis, a competing risk analysis using the Fine‐Grey model was used to analyse the risk of HF readmission.
We also analysed the echocardiographic data at discharge and 1 year after discharge. A multiple linear regression model was used to analyse the association between ePVS at discharge and the differences in EF, LVEDVI, and LVESVI at discharge and 1 year after discharge, after adjusting for patient age, sex, body mass index, hypertension, DM, chronic kidney disease (CKD), ACEis or ARBs or ARNIs, beta‐blockers, aldosterone antagonists, and peak CK at discharge. 14 , 15 , 16 , 17
The results are reported as hazard ratios (HRs), 95% confidence intervals (CIs), and P values. Statistical significance was set at P < 0.05. All statistical analyses were performed using the R software (version 3.1.2; R Foundation for Statistical Computing, Vienna, Austria).
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Results
Patients and patient characteristics
Of the patients enrolled in the NARA‐MI study, 1012 [excluding those who died during hospitalization (n = 80) or without measured ePVS at discharge (n = 3)] were included in the present study. Figure 1 shows the enrolment, exclusion criteria, and study flow.
The median age was 70 years (range, 61–78 years), and 76.4% of the patients were male. The high‐ePVS group (>5.5%) included 365 patients, and the low‐ePVS group (≤5.5%) included 647 patients (Table 1).
Table 1.
Baseline characteristics of the patients.
| Characteristics | All patients (N = 1012) | High‐ePVS group (N = 365) | Low‐ePVS group (N = 647) | P value |
|---|---|---|---|---|
| Age, years | 70 (61–78) | 76 (68–82) | 67 (58–74) | <0.001 |
| Male sex, % | 773 (76.4) | 209 (57.3) | 564 (87.2) | <0.001 |
| BMI, kg/m2 | 23.2 (20.9–25.6) | 21.8 (19.8–24.5) | 23.9 (21.8–26.2) | <0.001 |
| SBP, mmHg | 110 (102–122) | 110 (100–122) | 112 (102–124) | 0.124 |
| HR, beats/min | 70 (64–78) | 72 (65–78) | 70 (64–78) | 0.040 |
| Killip class III or IV, % | 112 (11.1) | 67 (18.4) | 45 (7.0) | <0.001 |
| STEMI | 835 (82.5) | 294 (80.5) | 541 (83.6) | 0.251 |
| Medical history, % | ||||
| Hypertension | 675 (66.7) | 263 (72.1) | 412 (63.7) | 0.008 |
| Dyslipidaemia | 552 (54.6) | 184 (50.4) | 368 (56.9) | 0.055 |
| Diabetes mellitus | 369 (36.5) | 142 (38.9) | 227 (35.1) | 0.253 |
| CKD | 481 (47.5) | 216 (59.2) | 265 (41.0) | <0.001 |
| Smoking | 382 (37.8) | 93 (25.5) | 289 (44.7) | <0.001 |
| Atrial fibrillation | 57 (5.6) | 23 (6.3) | 34 (5.3) | 0.581 |
| Myocardial infarction | 62 (6.1) | 30 (8.2) | 32 (4.9) | 0.051 |
| Prior PCI | 97 (9.6) | 41 (11.2) | 56 (8.7) | 0.220 |
| Prior CABG | 11 (1.1) | 5 (1.4) | 6 (0.9) | 0.737 |
| Cerebrovascular disease | 89 (8.8) | 46 (12.6) | 43 (6.6) | 0.002 |
| Peripheral arterial disease | 32 (3.2) | 15 (4.1) | 17 (2.6) | 0.265 |
| Medication at discharge, % | ||||
| ACEis or ARBs or ARNIs | 972 (96.1) | 342 (93.7) | 630 (97.4) | 0.007 |
| Beta‐blockers | 834 (82.4) | 291 (79.7) | 543 (83.9) | 0.110 |
| Aldosterone antagonists | 125 (12.4) | 65 (17.8) | 60 (9.3) | <0.001 |
| SGLT2 inhibitors | 76 (7.5) | 29 (7.9) | 47 (7.3) | 0.787 |
| Statins | 886 (87.6) | 298 (81.6) | 588 (90.9) | <0.001 |
| Ezetimibe | 158 (15.6) | 50 (13.7) | 108 (16.7) | 0.242 |
| Loop diuretics | 275 (27.2) | 131 (35.9) | 144 (22.3) | <0.001 |
| Loop diuretic dose, mg | 7.9 ± 15.2 | 10.5 ± 17.4 | 6.4 ± 13.7 | <0.001 |
| Culprit artery | ||||
| LMT | 20 (2.0) | 13 (3.6) | 7 (1.1) | 0.013 |
| LAD | 458 (45.3) | 167 (45.8) | 291 (45.0) | 0.863 |
| LCX | 123 (12.2) | 34 (9.3) | 89 (13.8) | 0.048 |
| RCA | 403 (39.8) | 146 (40.0) | 257 (39.7) | 0.984 |
| Multivessel disease | 316 (31.2) | 124 (34.0) | 192 (29.7) | 0.178 |
| Laboratory data at discharge | ||||
| Hb, g/dL | 12.4 (11.1–13.5) | 10.7 (9.9–11.3) | 13.1 (12.5–14.0) | <0.001 |
| Hct, % | 37.4 (33.8–40.6) | 32.7 (30.1–34.2) | 39.7 (37.7–42.1) | <0.001 |
| ePVS, mL/g | 5.1 (4.4–6.0) | 6.3 (5.9–7.0) | 4.6 (4.2–5.0) | <0.001 |
| Alb, g/dL | 3.7 (3.4–4.0) | 3.4 (3.2–3.7) | 3.8 (3.6–4.1) | <0.001 |
| HDL cholesterol, mg/dL | 38 (32–46) | 40 (33–48) | 37 (32–45) | <0.001 |
| LDL cholesterol, mg/dL | 80 (65–98) | 75 (63–90) | 83 (67–101) | <0.001 |
| TG, mg/dL | 113 (91–147) | 104 (84–131) | 119 (94–153) | <0.001 |
| HbA1c, % | 6.0 (5.3–6.9) | 5.9 (4.9–6.9) | 6.1 (5.5–7.0) | 0.302 |
| BUN, mg/dL | 16.0 (13.0–21.0) | 17.0 (13.0–25.0) | 15.0 (13.0–19.0) | <0.001 |
| Cr, mg/dL | 0.9 (0.8–1.1) | 0.9 (0.8–1.3) | 0.9 (0.8–1.0) | 0.037 |
| eGFR, mL/min/1.73 m2 | 61.2 (49.0–73.0) | 54.1 (37.0–70.8) | 63.5 (54.1–74.5) | <0.001 |
| Serum sodium, mEq/L | 140 (138–141) | 140 (137–141) | 140 (138–141) | 0.136 |
| Serum potassium, mEq/L | 4.3 (4.0–4.6) | 4.2 (3.9–4.5) | 4.3 (4.1–4.6) | <0.001 |
| Peak CK, U/L | 1843 (898–3,514) | 1,583 (789–3,164) | 2002 (999–3,733) | 0.001 |
| BNP, pg/mL | 197 (92–382) | 324 (199–601) | 139 (76–267) | <0.001 |
Note: Data are expressed as mean and standard deviation for normally distributed variables and median with interquartile range for non‐normally distributed variables. Categorical data are expressed as numbers and percentages.
Abbreviations: ACEis, angiotensin‐converting enzyme inhibitors; Alb, albumin; ARBs, angiotensin II receptor blockers; ARNIs, angiotensin receptor neprilysin inhibitors; BMI, body mass index; BNP, B‐type natriuretic peptide; BUN, blood urea nitrogen; CABG, coronary artery bypass grafting; CK, creatine kinase; CKD, chronic kidney disease; Cr, creatinine; eGFR, estimated glomerular filtration rate; ePVS, estimated plasma volume status; Hb, haemoglobin; HbA1c, haemoglobin A1c; Hct, haematocrit; HDL, high‐density lipoprotein; HR, heart rate; LAD, left anterior descending artery; LCX, left circumflex artery; LDL, low‐density lipoprotein; LMT, left main trunk; PCI, percutaneous coronary intervention; RCA, right coronary artery; SBP, systolic blood pressure; SGLT2, sodium‐glucose cotransporter 2; STEMI, ST‐elevation myocardial infarction; TG, triglycerides.
There were no significant differences between the groups in terms of systolic blood pressure, proportion of patients with STEMI, dyslipidaemia, DM, atrial fibrillation, history of MI, and prior PCI (Table 1). Age, proportion of males, Killip class III or IV, hypertension, CKD, and cerebrovascular disease were significantly higher in the high‐ePVS group than in the low‐ePVS group (Table 1).
In the low‐ePVS group, the proportions of patients treated with ACEis or ARBs or ARNIs and statins were significantly higher and those treated with aldosterone antagonists were significantly lower, compared with those in the high‐ePVS group. Both the proportion and dose of loop diuretics administered were significantly higher in the high‐ePVS group than in the low‐ePVS group (Table 1).
Regarding laboratory parameters at discharge, Hb, Hct, albumin, low‐density lipoprotein cholesterol, triglyceride, eGFR, and peak CK levels in the high‐ePVS group were significantly lower than those in the low‐ePVS group. BNP levels were significantly higher in the high‐ePVS group than in the low‐ePVS group (Table 1).
Clinical outcomes
During the median follow‐up period of 49.7 months, 179 all‐cause deaths (17.7%), 51 cardiovascular deaths (5.0%), and 51 HF readmissions (5.0%) occurred (Table 2). The Kaplan–Meier curve analyses showed that the high‐ePVS group had higher rates of all‐cause mortality, cardiovascular death, and HF readmission than the low‐ePVS group (log‐rank test, P < 0.001, respectively) (Figure 2). A competing risk analysis was performed to assess the effect of death, and a similar result was observed (Gray test, P < 0.001) (Figure S1).
Table 2.
Incidence of all‐cause death and HF readmission after discharge.
| High‐ePVS group (N = 365) | Low‐ePVS group (N = 647) | P value | |
|---|---|---|---|
| All‐cause death, % | 106 (29.0) | 73 (11.3) | <0.001 |
| Cardiovascular death, % | 33 (9.0) | 18 (2.8) | <0.001 |
| Infection, % | 10 (2.7) | 11 (1.7) | 0.377 |
| Malignancy, % | 19 (5.2) | 19 (2.9) | 0.099 |
| Others, % | 44 (12.1) | 25 (3.9) | <0.001 |
| HF readmission, % | 35 (9.6) | 16 (2.5) | <0.001 |
Note: P value refers to comparison of the proportions between the two groups using the Pearson's χ 2 test.
Abbreviations: ePVS, estimated plasma volume status; HF, heart failure.
Figure 2.
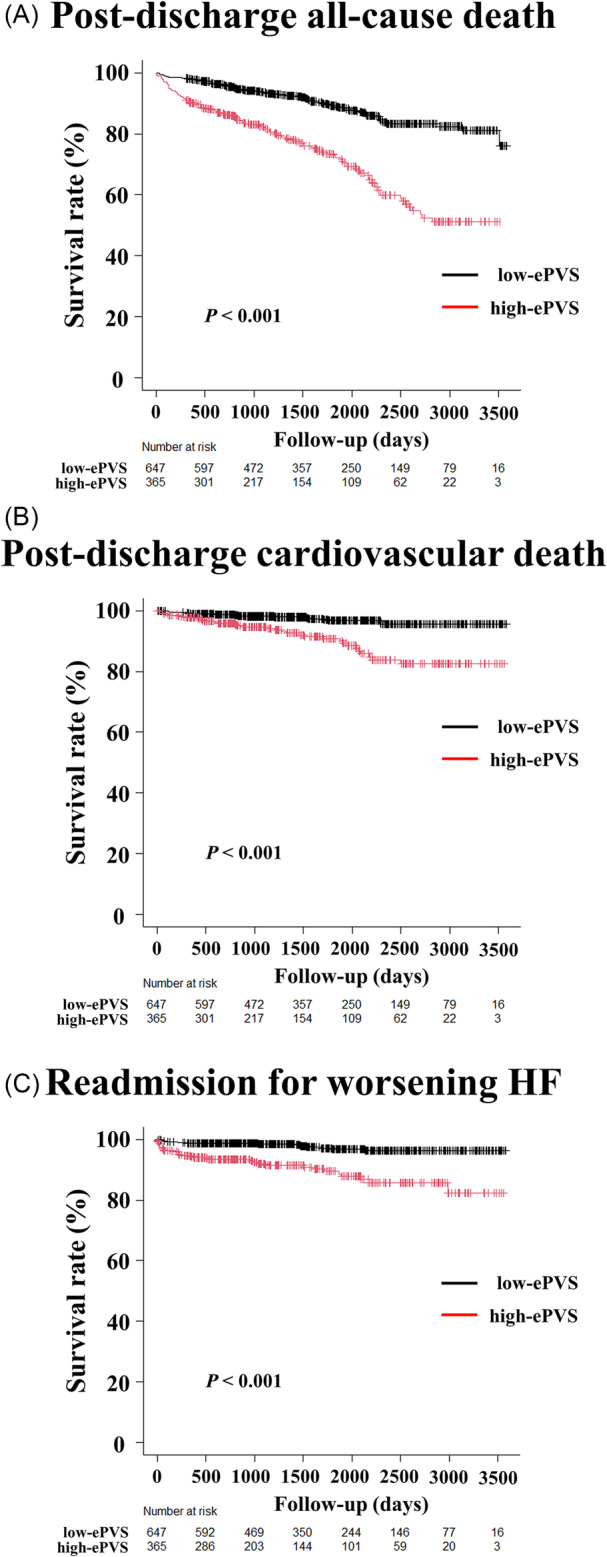
Kaplan–Meier analyses of ePVS at discharge for post‐discharge all‐cause mortality, cardiovascular death, and readmission for worsening HF. The Kaplan–Meier survival curves show the time to post‐discharge all‐cause death (A), cardiovascular death (B), and HF readmission (C) in the ePVS ≤ 5.5 (low‐ePVS) and ePVS > 5.5 (high‐ePVS) groups. The log‐rank test demonstrates that the high‐ePVS group has a significantly higher rate of all‐cause death, cardiovascular death, and HF readmission than the low‐ePVS group does (log‐rank test, P < 0.001) [HR, 3.116 (95% CI, 2.311–4.202; P < 0.001); HR, 3.879 (95% CI, 2.182–6.894; P < 0.001); HR, 4.599 (95% CI, 2.542–8.321; P < 0.001), respectively]. CI, confidence interval; ePVS, estimated plasma volume status; HF, heart failure; HR, hazard ratio.
In the univariate Cox regression analyses, the high‐ePVS group was associated with higher all‐cause mortality, compared with the low‐ePVS group (Table 3). In the multivariable Cox regression models adjusted for established prognostic factors for AMI (age, sex, Killip classification III or IV, hypertension, dyslipidaemia, DM, smoking, atrial fibrillation, peripheral artery disease, history of MI, history of stroke, multivessel disease, LVEF at discharge, eGFR, ACEis or ARBs or ARNIs, beta‐blockers, aldosterone antagonists, and statins at discharge), the high‐ePVS group was still associated with a higher all‐cause mortality, compared with the low‐ePVS group (HR, 1.879; 95% CI, 1.343–2.629; P < 0.001) (Table 3).
Table 3.
Cox regression analysis of outcomes in patients with AMI.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| ePVS > 5.5% | 3.116 (2.311–4.202) | <0.001 | 1.879 (1.343–2.629) | <0.001 |
| Age | 1.092 (1.074–1.109) | <0.001 | 1.077 (1.059–1.095) | <0.001 |
| Male sex | 0.931 (0.662–1.308) | 0.680 | 1.678 (1.132–2.487) | 0.010 |
| Killip III or IV classification | 2.680 (1.838–3.908) | <0.001 | 1.739 (1.151–2.627) | 0.009 |
| Hypertension | 1.091 (0.798–1.492) | 0.586 | 0.894 (0.639–1.249) | 0.510 |
| Dyslipidaemia | 0.504 (0.373–0.682) | <0.001 | 0.715 (0.519–0.984) | 0.039 |
| Diabetes mellitus | 1.158 (0.858–1.564) | 0.337 | 1.049 (0.765–1.439) | 0.765 |
| Smoking | 0.701 (0.511–0.961) | 0.027 | 1.243 (0.874–1.767) | 0.226 |
| Atrial fibrillation | 2.586 (1.604–4.168) | <0.001 | 1.431 (0.847–2.417) | 0.181 |
| Peripheral artery disease | 1.450 (0.595–3.537) | 0.414 | 0.857 (0.339–2.168) | 0.745 |
| History of myocardial infarction | 1.661 (0.994–2.777) | 0.053 | 0.981 (0.557–1.727) | 0.946 |
| History of stroke | 2.362 (1.576–3.539) | <0.001 | 1.194 (0.776–1.838) | 0.420 |
| Multivessel disease | 1.153 (0.844–1.575) | 0.370 | 1.069 (0.773–1.479) | 0.686 |
| LVEF at discharge | 0.954 (0.942–0.966) | <0.001 | 0.967 (0.953–0.981) | <0.001 |
| eGFR | 0.981 (0.975–0.988) | <0.001 | 1.00 (0.990–1.005) | 0.569 |
| ACEis or ARBs or ARNIs | 0.401 (0.237–0.681) | <0.001 | 0.714 (0.405–1.259) | 0.245 |
| Beta‐blockers | 0.650 (0.466–0.907) | 0.011 | 0.717 (0.507–1.015) | 0.061 |
| Aldosterone antagonists | 1.662 (1.148–2.404) | 0.007 | 0.838 (0.564–1.245) | 0.382 |
| Statins | 0.340 (0.247–0.468) | <0.001 | 0.654 (0.460–0.929) | 0.018 |
Abbreviations ACEis, angiotensin‐converting enzyme inhibitors; AMI, acute myocardial infarction; ARBs, angiotensin II receptor blockers; ARNIs, angiotensin receptor neprilysin inhibitors; CI, confidence interval; eGFR, estimated glomerular filtration rate; ePVS, estimated plasma volume status; HR, hazard ratio; LVEF, left ventricular ejection fraction.
Echocardiographic data
Among the echocardiographic parameters at discharge, the left ventricular (LV) end‐diastolic volume, E/A, and LVEF were significantly higher in the low‐ePVS group than in the high‐ePVS group. The left atrial volume index, left ventricular mass index, transtricuspid pressure gradient, and E/e′ were significantly higher in the high‐ePVS group than in the low‐ePVS group (Table 4 ).
Table 4.
Echocardiographic data at discharge.
| At discharge | |||
|---|---|---|---|
| High‐ePVS group (N = 287) | Low‐ePVS group (N = 568) | P value | |
| LAD, mm | 39.0 (35.0, 42.0) | 38.0 (35.0, 41.8) | 0.140 |
| LVEDD, mm | 47.0 (43.0, 51.0) | 48.0 (45.0, 51.0) | 0.011 |
| LVESD, mm | 32.0 (28.0, 37.0) | 32.0 (29.0, 36.0) | 0.358 |
| LVEDVI, mL/m2 | 48.4 (40.9, 59.4) | 48.8 (40.3, 58.6) | 0.450 |
| LVESVI, mL/m2 | 20.1 (15.1, 29.5) | 19.4 (15.1, 26.3) | 0.196 |
| LAVi, mL/m2 | 22.9 (17.9, 31.0) | 20.8 (15.1, 27.3) | <0.001 |
| LVMi, mL/m2 | 109.0 (91.6, 128.3) | 101.0 (86.5, 119.6) | <0.001 |
| TRPG, mmHg | 23.0 (18.8, 29.0) | 20.0 (17.0, 24.0) | <0.001 |
| E/A | 0.75 (0.62, 0.96) | 0.83 (0.67, 1.08) | <0.001 |
| E/e′ | 13.9 (10.8, 18.3) | 11.5 (9.1, 14.6) | <0.001 |
| LVEF, % | 57.0 (48.0, 64.0) | 59.8 (51.0, 64.0) | 0.042 |
Note: The difference between the two groups was tested using the Mann–Whitney U test for non‐normally distributed variables.
Abbreviations: E/A, early mitral inflow velocity; E/e′, early mitral inflow velocity to early diastolic mitral annular velocity; LAD, left atrial diameter; LAVi, left atrial volume index; LVEDD, left ventricular end‐diastolic diameter; LVEDVI, left ventricular end‐diastolic volume index; LVEF, left ventricular ejection fraction; LVESD, left ventricular end‐systolic diameter; LVESVI, left ventricular end‐systolic volume index; LVMi, left ventricular mass index; TRPG, tricuspid regurgitation pressure gradient.
Regarding changes in echocardiographic data between the time of discharge and 1 year after discharge, the low‐ePVS group showed greater increases in LVEF [2.3 (−1.7, 6.2)% vs. −0.4 (−4.6, 4.0)%] and decreases in LVEDVI [−3.7 (−11.2, 3.5) vs. −1.6 (−9.6, 9.1) mL/m2] and LVESVI [−2.5 (−6.4, 1.2) vs. −0.1 (−5.1, 5.0) mL/m2] than the high‐ePVS group (Figure 3). The results of the multiple linear regression analysis showed that low ePVS was significantly associated with increases in LVEF and decreases in LVEDVI and LVESVI in echocardiographic changes over 1 year (Tables S1–S3).
Figure 3.
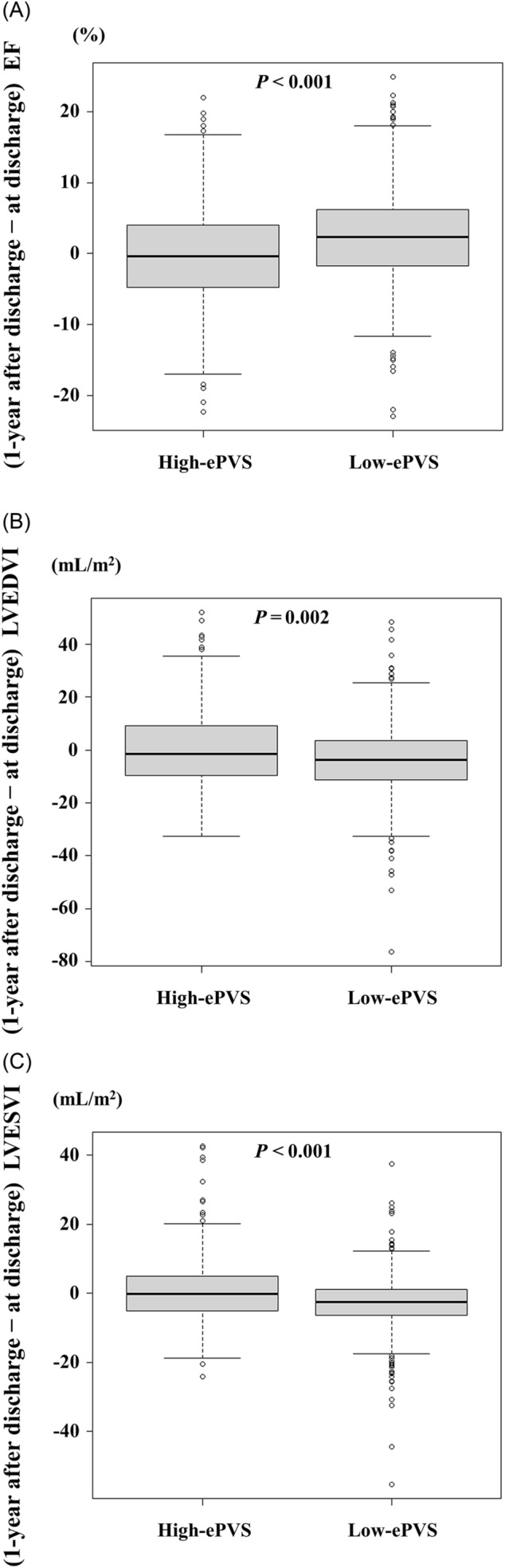
Relationship between ePVS and LV remodelling. The low‐ePVS group shows a greater 1 year‐increase in LVEF (A) and 1 year‐decrease in LVEDVI (B) and LVESVI (C) than the high‐ePVS group does ePVS, estimated plasma volume status; LV, left ventricular; LVEDVI, left ventricular end‐diastolic volume index; LVEF, left ventricular ejection fraction; LVESVI, left ventricular end‐systolic volume index.
Discussion
The present study examined the association between ePVS at discharge and the long‐term prognosis in patients with AMI. The main finding of the present study was that high ePVS at discharge was independently associated with high all‐cause mortality in patients with AMI. To the best of our knowledge, this is the first report to reveal that high ePVS at discharge is a strong prognostic predictor of long‐term outcomes in patients with AMI. Furthermore, low ePVS in AMI was significantly associated with increased LVEF and decreased LVEDVI and LVESVI 1 year after discharge. Thus, this study is also likely the first to report a significant association between low ePVS and LV reverse remodelling. These new findings indicate that low ePVS at discharge in patients with AMI may predict LV reverse remodelling 1 year after discharge. Thus, ePVS at discharge would be a useful indicator in clinical practice of patients with AMI.
Concomitant congestion in patients with AMI is a strong predictor of poor outcomes, and persistent congestion before discharge is associated with an increased risk of HF readmission and mortality. 18 , 19 Excessive dehydration leads to a decreased circulating PV, resulting in hypotension and multiple organ failure. Therefore, an accurate volume status assessment is important for improving congestive conditions. However, the procedure is difficult to perform in the clinical practice. This is because there is no single indicator for a congestive state. For example, assessing a clinical congestive state only in the presence or absence of symptoms may overlook the asymptomatic state of congestion.
ePVS (Strauss formula) can be calculated using a Hb‐ and Hct‐based formula. 6 Previous studies have shown a correlation between PV, total blood PV in the intravascular compartment, and ePVS. 6 , 20 In the present study, the values of BNP, transtricuspid pressure gradient, and E/e′ were significantly higher in the high‐ePVS group than in the low‐ePVS group. These findings suggest that high ePVS at discharge may represent asymptomatic haemodynamic congestion. Measuring ePVS for the assessment of congestion is non‐invasive, repeatable, and inexpensive, making it a practical and feasible indicator in the clinical setting. Therefore, many studies have assessed the prognostic impact of ePVS in patients with ADHF. 6 , 7 , 9 However, few studies have investigated whether ePVS at discharge is associated with long‐term prognosis in patients with AMI. The present study suggests that using ePVS at discharge as an indicator of congestion allows for appropriate PV control using diuretics and cardioprotective agents. This may lead to an improvement in the prognosis of patients with AMI.
The present study showed that high ePVS at discharge is an independent prognostic factor for high post‐discharge all‐cause mortality in patients with AMI. Moreover, low ePVS after adjusting for the covariates was significantly associated with increased LVEF 1 year after discharge and decreased LVEDVI and LVESVI 1 year after discharge. The reasons for these findings remain unclear; however, we believe that congestion and LV remodelling may have been involved. Congestion leads to increased LV wall stress, functional mitral regurgitation, and activation of neurohormonal and inflammatory pathways, which in turn contribute to adverse myocardial remodelling (chamber dilatation, increased ventricular sphericity, and aggravated ischaemia), loss of myocardial cells, reduced ventricular function, worsening haemodynamics, and progressive HF. 15 This is consistent with the fact that risk factors in patients with AMI, such as peak CK, age, sex, body mass index, hypertension, DM, CKD, and congestion, are involved in LV adverse remodelling, as well as the fact that cardioprotective drugs, such as ACEis, ARBs, ARNIs, beta‐blockers, and aldosterone antagonists, are involved in LV reverse remodelling. 14 , 15 , 16 , 17 Moreover, many reports have indicated that residual congestion is associated with poor prognosis in patients with HF. 3 , 21 Therefore, we believe that patients with AMI with high ePVS at discharge have residual congestion and that the effect of LV remodelling by increased LV wall stress and neurohormonal activation, which are caused by congestion, may lead to increased all‐cause mortality. These findings suggest that ePVS at discharge may be a novel prognostic marker in patients with AMI. A previous study showed that the use of loop diuretics was independently associated with an increased risk of all‐cause mortality and HF readmission in the low‐ePVS group but not in the high‐ePVS group. 22 Therefore, further studies with novel HF therapies, such as ARNIs and sodium‐glucose cotransporter 2 (SGLT2) inhibitors, are necessary to determine the appropriate treatment for patients with high ePVS.
This study has some limitations. First, this was a single‐centre study that involved a relatively small number of patients with AMI. Second, this was a retrospective analysis of prospectively collected data. Third, in the comparative analysis of echocardiographic data at discharge and 1 year after discharge, we had to exclude approximately 15% of patients owing to death and missing echocardiographic data at 1 year after discharge; therefore, the possibility of selection bias cannot be ruled out. Fourth, we could not directly evaluate the association between ePVS and volume status because we did not routinely perform right heart catheterization using the Swan–Ganz catheter during hospitalization. Fifth, to date, there is no established cut‐off value for ePVS that indicates the optimal PV status in patients with AMI. Our cut‐off value of 5.5 for ePVS has been established for patients with HF but needs to be confirmed for those with AMI in future studies. Sixth, ePVS (Strauss formula) is greatly influenced by Hb and Hct values. Therefore, the prognostic value of ePVS > 5.5% may be reduced in extreme anaemic conditions, and the appropriate cut‐off value of ePVS may vary depending on the degree of anaemia. Seventh, the lack of data on cardiac parameters such as BNP during the follow‐up period did not allow us to evaluate the association between these and ePVS at discharge. Finally, the occurrence of arrhythmia, such as atrial fibrillation and ventricular tachycardia, is a contributing factor to the progression of HF. However, the study did not include data on the occurrence of new arrhythmia, so it was impossible to determine the extent to which they contributed to HF exacerbations.
Conclusions
High ePVS at discharge was independently associated with high post‐discharge all‐cause mortality in patients with AMI. Our study suggests that ePVS at discharge in patients with AMI may be a novel prognostic marker. Further research is required to determine whether ePVS‐guided therapy improves the prognosis in patients with AMI.
Conflict of interest
Shungo Hikoso received research funds from Otsuka Pharmaceutical Co., Ltd., Bayer Yakuhin Ltd., Nippon Boehringer Ingelheim Co., Ltd., and Abbott Japan LLC. Yoshihiko Saito received research funds from Otsuka Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Actelion Pharmaceuticals Japan Ltd., Kyowa Kirin Co., Ltd., Dainippon Sumitomo Pharma Co., Ltd., Chugai Pharmaceutical Co., Ltd., Nihon Medi‐Physics Co., Ltd., and Fuji Yakuhin Co., Ltd.; research expenses from Roche Diagnostics K.K., Otsuka Pharmaceutical Co., Ltd., Terumo Corporation, Kowa Company, Ltd., Abbott Medical Japan LLC, Alnylam Japan K.K., and CMIC Holdings Co., Ltd.; speakers' bureau/honorarium from Alnylam Japan K.K., AstraZeneca K.K., Amicus Therapeutics, Inc., Amgen K.K. Edwards Lifesciences Corporation, Otsuka Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., Kowa Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Tsumura & Co., Toa Eiyo Ltd., Nippon Shinyaku Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma K.K., Bayer Yakuhin Ltd., Mochida Pharmaceutical Co., Ltd., and Janssen Pharmaceutical K.K.; consultation fees from AstraZeneca K.K., Novartis Pharma K.K., Nippon Boehringer Ingelheim Co., Ltd.; other remuneration (supervisor) from Towa Pharmaceutical Co., Ltd.
The other authors have no financial competing interests to disclose.
Funding
None.
Supporting information
Table S1. Multiple linear regression analysis of the differences in EF at discharge and 1 year after discharge.
Table S2. Multiple linear regression analysis of the differences in LVEDVI at discharge and 1 year after discharge.
Table S3. Multiple linear regression analysis of the differences in LVESVI at discharge and 1 year after discharge.
Figure S1. Supporting Information.
Nogi, K. , Ueda, T. , Nogi, M. , Ishihara, S. , Nakada, Y. , Hashimoto, Y. , Nakagawa, H. , Nishida, T. , Seno, A. , Onoue, K. , Watanabe, M. , Saito, Y. , and Hikoso, S. (2024) Prognostic value of estimated plasma volume status at discharge in acute myocardial infarction. ESC Heart Failure, 11: 3222–3231. 10.1002/ehf2.14912.
References
- 1. Bahit MC, Kochar A, Granger CB. Post‐myocardial infarction heart failure. JACC Heart Fail 2018;6:179‐186. doi: 10.1016/j.jchf.2017.09.015 [DOI] [PubMed] [Google Scholar]
- 2. Kawai T, Nakatani D, Yamada T, Sakata Y, Hikoso S, Mizuno H, et al. Clinical impact of estimated plasma volume status and its additive effect with the GRACE risk score on in‐hospital and long‐term mortality for acute myocardial infarction. Int J Cardiol Heart Vasc 2021;33:100748. doi: 10.1016/j.ijcha.2021.100748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rubio‐Gracia J, Demissei BG, ter Maaten J, Cleland JG, O'Connor CM, Metra M, et al. Prevalence, predictors and clinical outcome of residual congestion in acute decompensated heart failure. Int J Cardiol 2018;258:185‐191. doi: 10.1016/j.ijcard.2018.01.067 [DOI] [PubMed] [Google Scholar]
- 4. Hamilton E, Desta L, Lundberg A, Alfredsson J, Christersson C, Erlinge D, et al. Prevalence and prognostic impact of left ventricular systolic dysfunction or pulmonary congestion after acute myocardial infarction. ESC Heart Fail 2023;10:1347‐1357. doi: 10.1002/ehf2.14301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gheorghiade M, Filippatos G, De Luca L, Burnett J. Congestion in acute heart failure syndromes: an essential target of evaluation and treatment. Am J Med 2006;119:S3‐s10. doi: 10.1016/j.amjmed.2006.09.011 [DOI] [PubMed] [Google Scholar]
- 6. Kobayashi M, Girerd N, Duarte K, Chouihed T, Chikamori T, Pitt B, et al. Estimated plasma volume status in heart failure: clinical implications and future directions. Clin Res Cardiol 2021;110:1159‐1172. doi: 10.1007/s00392-020-01794-8 [DOI] [PubMed] [Google Scholar]
- 7. Nogi K, Ueda T, Nakamura T, Nogi M, Ishihara S, Nakada Y, et al. New classification for the combined assessment of the fractional excretion of urea nitrogen and estimated plasma volume status in acute heart failure. J Am Heart Assoc 2023;12:e025596. doi: 10.1161/JAHA.122.025596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol 2018;72:2231‐2264. doi: 10.1016/j.jacc.2018.08.1038 [DOI] [PubMed] [Google Scholar]
- 9. Duarte K, Monnez JM, Albuisson E, Pitt B, Zannad F, Rossignol P. Prognostic value of estimated plasma volume in heart failure. JACC Heart Fail 2015;3:886‐893. doi: 10.1016/j.jchf.2015.06.014 [DOI] [PubMed] [Google Scholar]
- 10. Kruck F, Bablok W, Besenfelder E, Betzien G, Kaufmann B. Clinical and pharmacological investigations of the new saluretic azosemid. Eur J Clin Pharmacol 1978;14:153‐161. doi: 10.1007/BF02089953 [DOI] [PubMed] [Google Scholar]
- 11. Diez J, Coca A, de Teresa E, Anguita M, Castro‐Beiras A, Conthe P, et al. TORAFIC study protocol: torasemide prolonged release versus furosemide in patients with chronic heart failure. Expert Rev Cardiovasc Ther 2009;7:897‐904. doi: 10.1586/erc.09.74 [DOI] [PubMed] [Google Scholar]
- 12. Hwang SY, Kim SH, Uhm IA, Shin JH, Lim YH. Prognostic implications for patients after myocardial infarction: an integrative literature review and in‐depth interviews with patients and experts. BMC Cardiovasc Disord 2022;22:348. doi: 10.1186/s12872-022-02753-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johansson S, Rosengren A, Young K, Jennings E. Mortality and morbidity trends after the first year in survivors of acute myocardial infarction: a systematic review. BMC Cardiovasc Disord 2017;17:53. doi: 10.1186/s12872-017-0482-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Konstam MA, Kramer DG, Patel AR, Maron MS, Udelson JE. Left ventricular remodeling in heart failure: current concepts in clinical significance and assessment. JACC Cardiovasc Imaging 2011;4:98‐108. doi: 10.1016/j.jcmg.2010.10.008 [DOI] [PubMed] [Google Scholar]
- 15. Parrinello G, Greene SJ, Torres D, Alderman M, Bonventre JV, Di Pasquale P, et al. Water and sodium in heart failure: a spotlight on congestion. Heart Fail Rev 2015;20:13‐24. doi: 10.1007/s10741-014-9438-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gjesdal O, Bluemke DA, Lima JA. Cardiac remodeling at the population level—risk factors, screening, and outcomes. Nat Rev Cardiol 2011;8:673‐685. doi: 10.1038/nrcardio.2011.154 [DOI] [PubMed] [Google Scholar]
- 17. Bhatt AS, Ambrosy AP, Velazquez EJ. Adverse remodeling and reverse remodeling after myocardial infarction. Curr Cardiol Rep 2017;19:71. doi: 10.1007/s11886-017-0876-4 [DOI] [PubMed] [Google Scholar]
- 18. Sulo G, Igland J, Nygard O, Vollset SE, Ebbing M, Poulter N, et al. Prognostic impact of in‐hospital and postdischarge heart failure in patients with acute myocardial infarction: a nationwide analysis using data from the Cardiovascular Disease in Norway (CVDNOR) project. J Am Heart Assoc 2017;6:6. doi: 10.1161/JAHA.116.005277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chioncel O, Mebazaa A, Harjola VP, Coats AJ, Piepoli MF, Crespo‐Leiro MG, et al. Clinical phenotypes and outcome of patients hospitalized for acute heart failure: the ESC Heart Failure Long‐Term Registry. Eur J Heart Fail 2017;19:1242‐1254. doi: 10.1002/ejhf.890 [DOI] [PubMed] [Google Scholar]
- 20. Dekkers CCJ, Sjostrom CD, Greasley PJ, Cain V, Boulton DW, Heerspink HJL. Effects of the sodium‐glucose co‐transporter‐2 inhibitor dapagliflozin on estimated plasma volume in patients with type 2 diabetes. Diabetes Obes Metab 2019;21:2667‐2673. doi: 10.1111/dom.13855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ambrosy AP, Pang PS, Khan S, Konstam MA, Fonarow GC, Traver B, et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J 2013;34:835‐843. doi: 10.1093/eurheartj/ehs444 [DOI] [PubMed] [Google Scholar]
- 22. Kawai T, Nakatani D, Watanabe T, Yamada T, Morita T, Sakata Y, et al. Loop diuretic use is associated with adverse clinical outcomes in acute myocardial infarction patients with low volume status. Curr Probl Cardiol 2022;47:101326. doi: 10.1016/j.cpcardiol.2022.101326 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Multiple linear regression analysis of the differences in EF at discharge and 1 year after discharge.
Table S2. Multiple linear regression analysis of the differences in LVEDVI at discharge and 1 year after discharge.
Table S3. Multiple linear regression analysis of the differences in LVESVI at discharge and 1 year after discharge.
Figure S1. Supporting Information.


