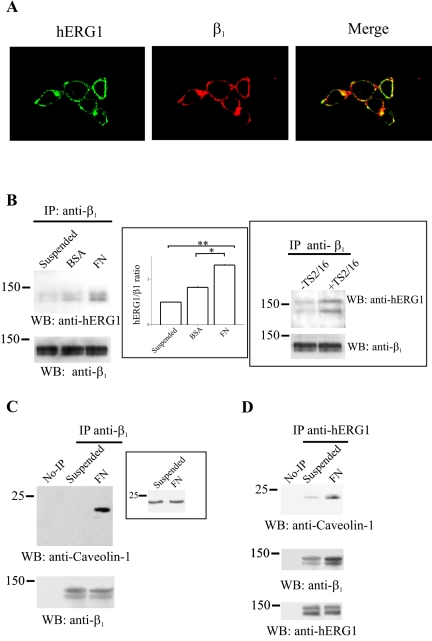
Figure 4.
Characterization of the β1/hERG1 complex in HEK-hERG1 cells. (A) Colocalization of hERG1 and β1 proteins. Double immunofluorescence was performed on HEK-hERG1 cells seeded onto glass slides coated with 20 μg/ml FN in DMEM + BSA, by using anti-hERG1 antibodies (C54) and a FITC-conjugated secondary antibody (hERG1) and anti-β1 antibodies (TS2/16) and a RITC-conjugated secondary antibody (β1). Photographs were taken with a confocal microscope and merged (merge). A quantitative analysis of confocal images, using the MetaMorph Software (integrated morphometry analysis; Universal Imaging) showed that 18% (±2, n = 12) of the hERG1 protein and of β1 integrin colocalize in HEK-hERG1 cells. (B) CoIP of β1 and hERG1 proteins depends on β1 activity. CoIP experiments were performed on cells kept in suspension (lane suspended) or seeded either onto BSA- (lane BSA)- or FN (lane FN)-coated dishes for 30 min, by using anti-β1 antibodies (TS2/16) to immunoprecipitate and anti-hERG1 antibodies for the Western blot. Reprobing with anti-β1 antibodies also is reported in the bottom panel. Inset (center), densitometric analysis of the amount of hERG1 protein that coimmunoprecipitates with β1 after cell adhesion on different substrates. The analysis was performed as described in Materials and Methods; data are the means ± SEM of three separate experiments. *, significantly different p = 0.00016, Student's t test (FN adherent cells vs. BSA adherent cells); **, significantly different p = 0.0000016, Student's t test (FN adherent cells vs. suspended cells). Inset (right), HEK-hERG1 cells were kept in suspension and treated (lane +TS2/16) or not (lane –TS2/16) for 30 min with activating monoclonal anti-β1 antibodies as described in Materials and Methods. CoIP experiments were performed as described above. (C and D) Colocalization of β1 with caveolin-1 and hERG1 proteins. Proteins were extracted from HEK-hERG1 cells kept in suspension (lane suspended) or seeded onto FN (lane FN)-coated dishes for 30 min and immunoprecipitated using anti-β1 antibodies (TS2/16); no-IPs refer to samples where no primary antibody was added to cell lysates, as described in Materials and Methods. Bands were revealed using anti-caveolin-1 antibodies. Reprobing with anti-β1 antibodies also is reported at the bottom. Inset, total cell lysate obtained from cells treated as in C probed with anti-caveolin-1 antibodies. (D) CoIP of hERG1, caveolin-1 and β1 proteins. Proteins were extracted from HEK-hERG1 cells kept in suspension (lane suspended) or seeded onto FN-coated dishes for 30 min (lane FN), and immunoprecipitated using anti-hERG1 antibodies (N135); bands were revealed using anti-caveolin-1 antibodies (top), anti-β1 antibodies (middle), and anti-hERG1 antibodies (C54) (bottom). All the panels in this figure are representative of at least three different experiments, except for TS2/16 treatment (inset to B, right), which was a single experiment. No-IP, no immunoprecipitation.