Figure 1.
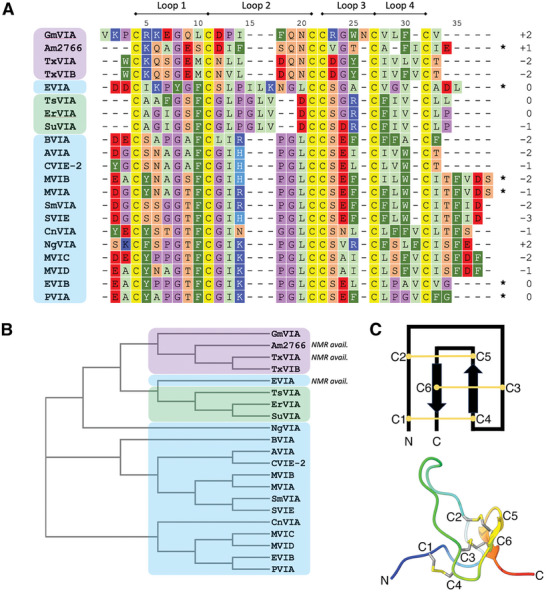
A) Sequence alignment of the δ‐conotoxins modeled in this study, together with the overall net charge and loop nomenclature. Peptides from piscivorous, vermivorous, and molluscivorous cone snails are highlighted in blue, green, and purple, respectively. C‐terminal amides are indicated with *. B) Phylogenetic analysis of the sequences shown in A indicating peptides with available experimental structures and colored according to the color scheme in A. C) (top) Features typical of an inhibitor cystine knot (ICK) peptide, showing the connectivity of the disulfide bonds (numbering refers to the order in which cysteines appear, not residue numbering) and (bottom) One conformation of the NMR‐derived structure of δ‐EVIA (PDB ID: 1G1P)[ 13 ] showing the classic ICK fold.
