Figure 5.
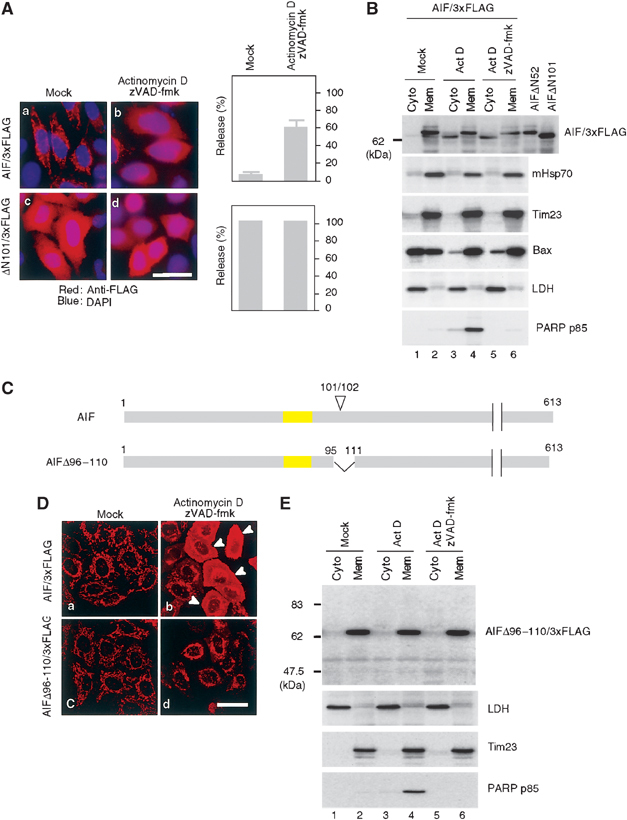
Processing of AIF is essential for the mitochondrial release by proapoptotic stimuli. (A) Subcellular distribution of full-length AIF/3xFLAG and ΔN101AIF/3xFLAG lacking the IMS localization signal. HeLa cells expressing the indicated constructs were treated with or without 20 μM actinomycin D in the presence of zVAD-fmk. The cells were stained with anti-FLAG antibodies (red) and DAPI (blue), and examined by fluorescence confocal microscopy. The number of cells exhibiting cytoplasmic patterns for each construct was counted and is shown in the right panel. Other conditions are same as in (B). (B) HeLa cells expressing AIF/3xFLAG were treated as described in (A), and then fractionated into membrane and cytosolic fractions, and each fraction was analyzed by immunoblotting using antibodies against the indicated proteins. (C) Schematic representation of AIF/3xFLAG and the truncation mutant. TMS is shown in yellow boxes and apoptosis-dependent processing site (101/102) is indicated on the top of the figure. (D) Subcellular distribution of full-length AIF/3xFLAG and AIFΔ96–110/3xFLAG. (E) HeLa cells expressing AIFΔ96–110/3xFLAG were treated with actinomycin D in the presence or absence of zVAD-fmk. They were fractionated into membrane and cytosolic fractions, and each fraction was analyzed by immunoblotting using antibodies against the indicated proteins.
