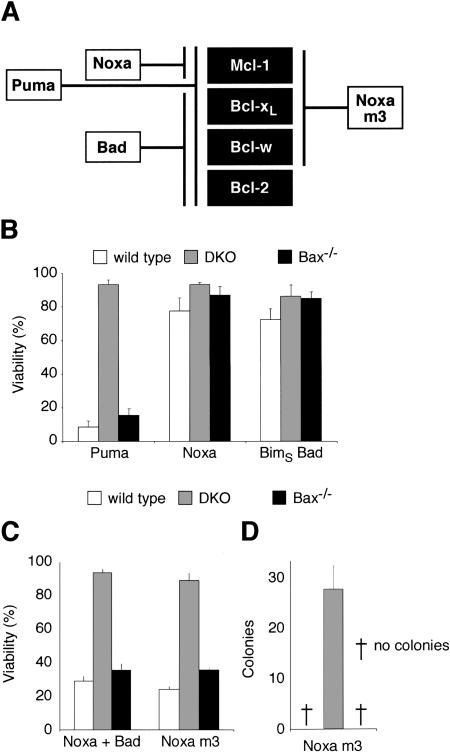
Figure 6.
Neutralization of Mcl-1 and Bcl-xL triggers Bak-dependent apoptosis. (A) Selective binding profiles of Bad, Noxa, and Noxa m3, based on interaction studies (Chen et al. 2005). Puma binds all prosurvival proteins tested; Bad binds tightly to Bcl-xL, Bcl-w, and Bcl-2, whereas Noxa selectively targets Mcl-1. In addition to Mcl-1, Noxa m3 also binds Bcl-xL and Bcl-w, but its affinity for Bcl-2 is insignificant (>10,000 nM). (B) Puma, but not Noxa or BadBH3, is sufficient to induce Bak-mediated apoptosis. Wild-type MEFs, Bax and Bak doubly deficient MEFs (DKO), or MEFs lacking only Bax were infected with the indicated retroviruses. The BadBH3 was tested within an inert BimS backbone (Chen et al. 2005) to preclude any effects due to regulation of the Bad protein. Expression of each BH3-only protein was linked via an IRES to that of GFP, and the viability of GFP+ve cells was determined by PI exclusion 24 h after infection. (C) The weak killing activity of Noxa, which only targets Mcl-1, can be complemented by neutralization of Bcl-xL. The indicated MEFs were infected with retroviruses coexpressing Noxa and BimSBadBH3 (Chen et al. 2005). The combination of the BadBH3 (which neutralizes Bcl-2, Bcl-xL, and Bcl-w; see A) and Noxa gives potent Bak-dependent killing. Retroviral infection with Noxa m3 caused comparable killing of wild-type MEFs and those only expressing Bak. (A) As Noxa m3 binds Mcl-1, Bcl-xL, and Bcl-w but not Bcl-2, targeting of these prosurvival proteins suffices for Bak-mediated apoptosis, whereas neutralization of Bcl-2 is not required. (D) Bcl-2 is not required for killing by Noxa m3 in long-term colony assays. Equivalent numbers of retrovirally infected cells were plated and the number of colonies formed scored 6 d later. Data in B-D represent mean ± SD from three independent experiments.