Figure 5.
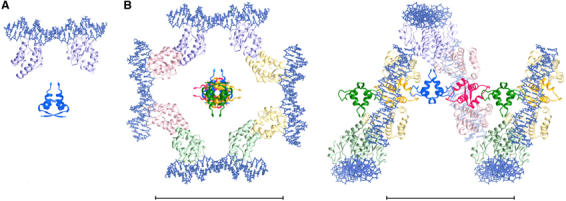
A model of the interactions between a SeqA filament and DNA. (A) A SeqA–N dimer and a pair of crystallographic SeqA–C–DNA complexes are placed to share a common dyad axis. The protein is shown as ribbon diagrams in dark (SeqA–N) and light (SeqA–C) blue and the DNA depicted as stick models. (B) The full-length SeqA dimer–DNA model is allowed to multimerize according to the 43 screw axis of the SeqA–N filament. The four-fold screw axis is perpendicular to the plane. Each SeqA–N dimer and the SeqA–C pair bound to it are shown in dark and light shades of a distinct color. Space between the N- and C-terminal domains accounts for the flexible linker and avoids contacts or clashes between neighboring SeqA–C molecules related by the 43 screw axis. An orthogonal view placing the SeqA–DNA superhelix in plane is shown on the right panel. No artificial coordinates are introduced, and DNAs are left to be discontinuous between adjacent SeqA dimers. Scale bars represent 100 Å.
