Figure 5.
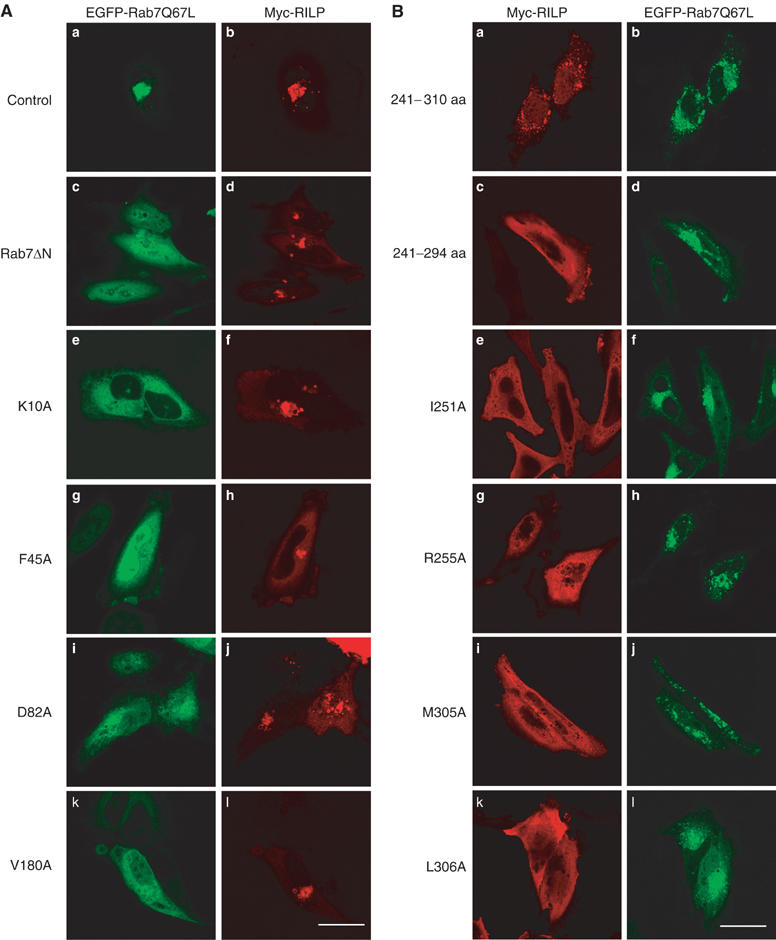
Effects of mutations of Rab7 and RILP on their cellular localization and membrane recruitment. (A) Representative site-directed mutants (panels e, g, i and k) or a truncated form (Rab7ΔN; panel c) of Rab7Q67L defective in interaction with RILP are mistargeted to the cytosol (and nucleus for F45A) (as revealed by GFP attached to the N-terminus of these proteins) and are not detected in the clustered lysosomes marked by coexpressed RILP (panels d, f, h, j and l as revealed by Myc tag). EGFP-Rab7Q67L (panel a) and Myc-RILP (panel b) serve as the positive control. Bar, 20 μm. (B) Representative mutants of RILP (panels c, e, g, i and k, revealed by Myc tag) defective in interaction with Rab7 are mistargeted to the cytosol and did not associate with punctuate late endosomes/lysosomes marked by coexpressed EGFP-Rab7Q67L (panels b, d, f, h, j and l, viewed by GFP). The fragment encompassing residues 241–310 of RILPe is peripheral distributed, but still can be efficiently recruited to the punctuate structures marked by EGFP-Rab7Q67L (panels a and b). Bar, 20 μm.
